$7.5 million to study elusive cell type important in aging, cancer, other diseases
Elusive senescent cells are subject of new national research consortium
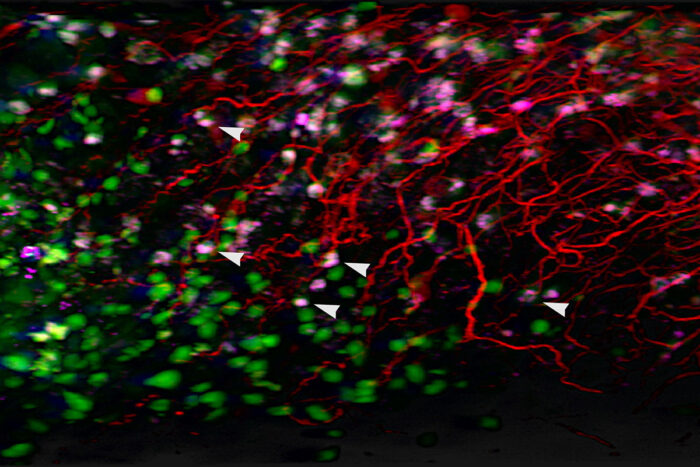 Nataly Naser AL Deen, Feng Chen
Nataly Naser AL Deen, Feng ChenWashington University School of Medicine in St. Louis has joined the NIH's SenNet, a national research network focused on understanding senescent cells, an elusive but important cell type that plays key roles in the diseases of aging. Pictured above, the white arrows point to senescent cells identified in the stomach tissue of an older mouse using 3D lightsheet microscopy.
Washington University School of Medicine in St. Louis is joining the National Institutes of Health’s (NIH) new research network focused on the study of senescent cells, a rare and important population of cells that is difficult to study but vital for understanding aging and the diseases of aging, including cancer and neurodegeneration. The goal is to help researchers develop new therapies that target cellular senescence to prevent or treat such diseases and improve human health.
Washington University will receive $7.5 million over five years to support the research.
The Cellular Senescence Network (SenNet) is supported by the NIH Common Fund and overseen by the National Institute on Aging (NIA) and the National Cancer Institute (NCI). The NIH will provide a total of about $125 million to 16 research centers over five years, pending available funds. The NIH has plans to expand SenNet through additional awards in the future.
The NIH is establishing SenNet to identify and describe senescent cells across multiple tissues throughout the body, in various states of health and disease, and at many ages across the human life span. Harnessing tissue samples from model organisms and people, the national network will provide freely accessible atlases of senescent cells, including information about the differences among them and the molecules they produce.
Washington University School of Medicine will serve as one of eight tissue-mapping centers. With a focus on bone marrow and liver, Washington University researchers will map the location and function of senescent cells in many samples of these specific tissues. Such research could shed light on the development of cancers of the blood and liver as well as metabolic disorders such as fatty liver disease.
The Washington University Senescence Tissue Mapping Center (WU-SN-TMC) will be led by principal investigator Li Ding, PhD, a professor of medicine and of genetics; and co-principal investigators Feng Chen, PhD, an associate professor of medicine; Ryan C. Fields, MD, the Kim and Tim Eberlein Distinguished Professor of Surgery; and Sheila A. Stewart, PhD, the Gerty Cori Professor of Cell Biology & Physiology and a professor of medicine.
“We are excited and proud to be a part of this national effort to understand cellular senescence,” Ding said. “These cells are quite mysterious. We don’t have good ways of detecting senescent cells in the body. They have lost their ability to divide, and some think that they are more fragile than normal cells. They also secrete a characteristic set of proteins as part of the ‘senescence associated secretory phenotype.’ Senescent cells are not cancerous, but they can lead to inflammation that sets the stage for cancer to develop along with other diseases that we associate with aging. Our group will map out senescent cells in bone marrow and liver samples in an effort to understand their spatial distribution and molecular signature in different tissue environments and at different ages.”
Senescent cells have stopped dividing and do not regain the ability to divide again. For years, scientists suspected that cellular senescence only happened in cells growing in a laboratory culture dish and that such cells did not exist in living organisms. Improved technologies have proven this wrong, but the cells remain difficult to study in their natural environments. Much of the consortium’s work will be focused on developing better ways to identify these elusive cells and new laboratory tools to study them. Such tools will build upon previous advances in single-cell analysis, such as those from the Common Fund’s Human Biomolecular Atlas Program and Single Cell Analysis Program.
According to the NIH, SenNet aims to unite cellular senescence researchers by creating common terms and classifications for senescent cells. SenNet will provide data and resources to the public that would otherwise be difficult to achieve through individual efforts, accelerating the ability of biomedical researchers to develop therapeutics that target cellular senescence and improve human health.
“The study of senescent cells holds great promise in helping to assess the role of these cells in the development of age-related disease, including cancer,” said Norman E. “Ned” Sharpless, MD, director of the NIH’s National Cancer Institute. “Cancer is certainly one of the most prevalent chronic age-related diseases. We look forward to the potential translational benefits of this research in answering some of the most challenging questions about aging and cancer. This initiative will advance our work in the cancer research field overall.”
The other tissue-mapping centers include Yale University, the Buck Institute for Research on Aging in Novato, Calif., the University of Pittsburgh, the University of Connecticut, Duke University, the University of Minnesota and Columbia University. The network also includes a data coordinating center at the University of Pittsburgh and technology development centers at Stanford University, Massachusetts General Hospital, the University of Washington, the University of Michigan, the Buck Institute for Research on Aging, Brown University and the Mayo Clinic in Rochester, Minn.






