$8 million awarded to study root causes of brain cell death in fatal pediatric diseases
Team science approach leverages expertise of researchers from diverse fields
 Sara Moser
Sara Moser A large, multidisciplinary team at Washington University School of Medicine in St. Louis has received a grant from the National Institute of Neurological Disorders and Stroke to tackle complex problems in neuroscience.
A large team of researchers at Washington University School of Medicine in St. Louis has received nearly $8 million from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH) to help determine the root causes of brain cell death in fatal pediatric neurodegenerative diseases.
The grant is part of the inaugural Collaborative Opportunities for Multidisciplinary, Bold, and Innovative Neuroscience (COMBINE) program, which provides funding to groups of investigators from diverse fields to tackle complex problems in neuroscience that exceed the limitations of a single laboratory. Six teams committed to collaborating, crossing technical and conceptual boundaries, and promoting equal opportunities for involved researchers and study participants receive funding support for five years.
“We know the neurons in young patients with neurodegenerative diseases die, but we don’t clearly understand why,” said Marco Sardiello, PhD, an associate professor of pediatrics at the School of Medicine. “We want to map the pathways that eventually cause cell death as a possible entry point for developing therapies. Pathways are composed of interacting proteins and molecules, and many of them, in theory, could be leveraged to devise therapeutic strategies. But we need to define these molecules first, or there is no clear path forward.”
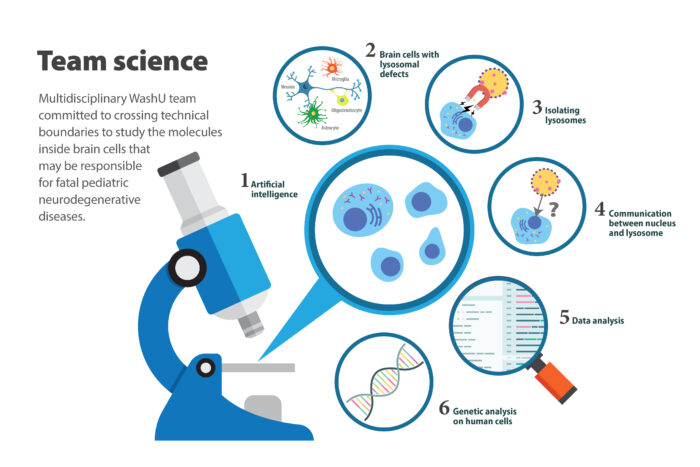
The new grant brings together scientists in pediatrics, genetics, bioinformatics and psychiatry to understand how and why cells die in two lysosomal storage disorders (LSD) that affect the brain. LSDs include over 50 genetic diseases that individually are rare, but collectively are prevalent and affect different parts of the body. The two LSDs the researchers will study – Sanfilippo syndrome and Late Infantile Neuronal Ceroid Lipofuscinosis – affect children who appear healthy until they reach toddlerhood and start to regress in developmental milestones, losing more and more skills over time. Intellectual and cognitive function also decline, as does muscle coordination, often leading to death in adolescence. There are no cures, and therapies to slow the progress of the disorders are limited and invasive. The funded research aims to lay a foundation for the development of therapies that could potentially halt brain cell death.
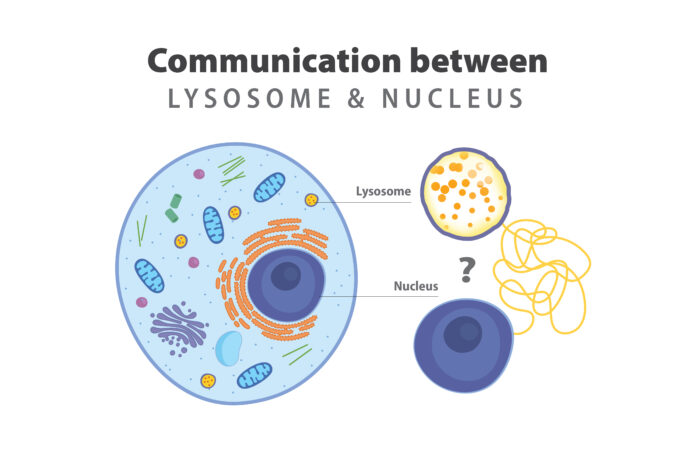
For decades, the prevailing explanation of cell death in lysosomal storage disorders has not changed. The stomach of the cell — the lysosome — unable to digest cellular waste, accumulates debris until the cell becomes too full to function and dies. However, there is evidence in the field that doesn’t satisfy this paradigm, hinting at the possibility that the prevailing theory is only partially true.
Patricia I. Dickson, MD, the Centennial Professor of Pediatrics and director of the Division of Genetics & Genomic Medicine in the Department of Pediatrics, recruited Jonathan D. Cooper, PhD, a professor of pediatrics, and Sardiello to Washington University in 2018 and 2020, respectively. Brainstorming sessions during their respective job interviews set the stage for taking on the team science approach.
They first secured seed funding from the NeuroGenomics and Informatics Center, which is led by Carlos Cruchaga, PhD, a professor of psychiatry, by writing a pilot project that also included William Buchser, PhD, an associate professor of genetics and director of the Functional Imaging for Variant Elucidation core facility in the McDonnell Genome Institute. They added Cruchaga and Fuhai Li, PhD, an assistant professor of pediatrics, to the team, and together they started to refine the lines of research and collect data. When the NIH’s new funding opportunity asked for innovative research challenging existing paradigms, the timing was perfect.
According to the research team, a communication failure between the lysosome and the command center of the cell, the nucleus, is an intriguing possibility for what goes wrong and causes brain cell death. The researchers have cutting-edge tools to identify molecules in the lysosomes that may be giving the wrong message to a protein that communicates between lysosomes and genes inside the nucleus. The team questions whether these molecules may be an underlying cause of lysosomal storage diseases.
 Sara Moser
Sara Moser“We have the tools to determine which brain cells – neurons, microglia, oligodendrocytes and astrocytes – contribute to disease outcome in Sanfilippo syndrome and for Late Infantile Neuronal Ceroid Lipofuscinosis,” Cooper said.
They also plan to use artificial intelligence systems to evaluate cells’ features – similar to how the brain enables quick recognition of familiar faces. Because they can define a sick cell by evaluating its unique features, they can then perturb the system by altering any novel pathways they discover and ask how that affects the health of the cell – does it make it better or worse?
The researchers also will analyze mice and human cell samples from children with lysosomal storage diseases. Patients travel from throughout the region to seek care at Washington University, where pediatric geneticists specialize in treating children with these diseases. The researchers are committed to recruiting diverse study participants by working with nonprofit organizations supporting affected families. To include children from underrepresented communities, they will engage with these communities to raise awareness.
 Sara Moser
Sara MoserThe project’s other investigators are: Yun Ju Sung, PhD, an associate professor of psychiatry and biostatistics; Philip R.O. Payne, PhD, the Janet and Bernard Becker Professor, director of the Institute for Informatics, Data Science and Biostatistics, associate dean for health information and data science, and chief data scientist at the School of Medicine; Jaiprakash Sharma, PhD, an instructor in pediatrics; Abhinav Diwan, MD, a professor of medicine, cell biology & physiology, and obstetrics & gynecology; and Young Ah Goo, PhD, a professor of biochemistry and molecular biophysics, genetics, and director of the Mass Spectrometry Technology Access Center in the McDonnell Genome Institute. The team also will collaborate with Giuseppe Martano, PhD, co-head of the Metabolomics Core Facility of Humanitas Research Hospital in Italy.
Per the NINDS’ Plan for Enhancing Diverse Perspectives requirement, the team aims to amplify every team member’s voice, including trainees, junior investigators and individuals at all career stages.
“Through this interdisciplinary team science grant, everyone contributes equally,” Dickson said. “We truly need the integration of different disciplines and tools, because no one lab can accomplish what we aim to do. We need everyone’s novel ideas and unique perspectives to achieve this.”






