Brain network responsible for Parkinson’s disease identified
Patients receiving treatments targeted to the network, rather than to nearby brain regions, experience larger improvements
 Sara Moser/WashU Medicine
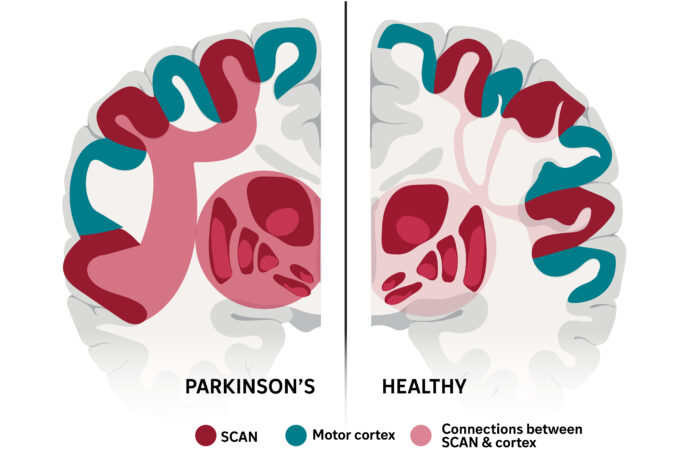
Sara Moser/WashU MedicineThe brain network that links thinking with movement, called SCAN, was first described by WashU Medicine researchers in 2023 and has been identified in a new study as the neurological basis of Parkinson’s disease. An experimental therapy that targeted this network more than doubled symptom improvement in a small group of patients with Parkinson’s, which is characterized by hyperconnectivity (left side of illustration) between SCAN and the brain’s subcortex.
Parkinson’s disease, a progressive neurological disorder affecting more than 1 million people in the U.S. and more than 10 million globally, is characterized by debilitating symptoms such as tremors, movement difficulties, sleep disturbances and cognitive impairments. While current treatments, including long-term medication and invasive deep brain stimulation (DBS), can alleviate symptoms, they cannot halt progression or cure the disease.
A new international study led by China’s Changping Laboratory, in collaboration with Washington University School of Medicine in St. Louis and others, identifies the region of the brain responsible for the core problems of Parkinson’s disease. Targeting this brain network — the somato-cognitive action network (SCAN) — with a non-invasive, experimental therapy called transcranial magnetic stimulation (TMS) more than doubled the improvement in symptoms in a small group of patients, compared to TMS acting upon surrounding brain areas.
The study, published Feb. 4 in Nature, redefines the neurological basis of Parkinson’s and lays the groundwork for more-effective, precision treatment of the disease.
“This work demonstrates that Parkinson’s is a SCAN disorder, and the data strongly suggest that if you target the SCAN in a personalized, precise manner you can treat Parkinson’s more successfully than was previously possible,” said co-author Nico U. Dosenbach, MD, PhD, the David M. & Tracy S. Holtzman Professor of Neurology at WashU Medicine. “Changing the activity within SCAN could slow or reverse the progression of the disease, not just treat the symptoms.”
Treating the root of Parkinson’s disease
Dosenbach first described SCAN in Nature in 2023. The network lies within the motor cortex — the part of the brain that controls body movements — and is responsible for turning action plans into movements and receiving feedback on how executing those plans went. Given that Parkinson’s disease causes a broad range of symptoms, affecting bodily functions such as movement, digestion and sleep as well as cognition and motivation, Hesheng Liu, PhD, the study’s senior author, teamed up with Dosenbach to explore whether dysfunction of SCAN, which links cognition with movement, could explain Parkinson’s disease symptoms and serve as a target for treatment.
Liu’s team collected various brain imaging data from more than 800 participants across multiple institutions in the U.S. and China. The group included patients with Parkinson’s disease receiving DBS, which uses surgically implanted electrodes to send electrical impulses to specific brain areas, or non-invasive treatments such as transcranial magnetic stimulation, focused ultrasound stimulation and medications. There were also healthy individuals and patients with other movement disorders included as controls.
The authors’ analysis revealed that Parkinson’s disease is characterized by hyperconnectivity between the SCAN and the subcortex, the part of the brain responsible for emotion, memory and motor control. All four therapies included in the study were most effective when they reduced hyperconnectivity between the SCAN and the subcortex, ultimately normalizing activity in the circuit responsible for planning and coordinating action.
“For decades, Parkinson’s has been primarily associated with motor deficits and the basal ganglia,” the part of the brain that controls muscle movements, Liu said. “Our work shows that the disease is rooted in a much broader network dysfunction. The SCAN is hyperconnected to key regions associated with Parkinson’s disease, and this abnormal wiring disrupts not only movement but also related cognitive and bodily functions.”
Leveraging this insight, the researchers developed a new precision treatment system capable of targeting the SCAN non-invasively with millimeter accuracy. They applied transcranial magnetic stimulation, which sends magnetic pulses to the brain from a device on the head. In a clinical trial, 18 patients receiving SCAN-targeted transcranial magnetic stimulation showed a 56% response rate after two weeks, compared to 22% in a group of 18 patients receiving stimulation at adjacent brain areas — a 2.5-fold increase in efficacy.
“With non-invasive treatments, we could start treating with neuromodulation much earlier than is currently done with DBS” because they don’t require brain surgery, said Dosenbach.
Dosenbach added that there’s more basic research to be done to understand if and how different components of the SCAN affect different Parkinson’s symptoms.
Dosenbach is planning clinical trials with Turing Medical, a WashU Medicine startup he co-founded, to test a non-invasive treatment using surface electrode strips placed over SCAN regions to treat gait dysfunction in Parkinson’s patients. He also plans to investigate modulating the SCAN with low-intensity focused ultrasound, a non-invasive way to change brain activity using acoustic energy.