Drug compound makes pancreatic cancer cells more vulnerable to chemo
Mouse studies show promise for potential new pancreatic cancer therapy
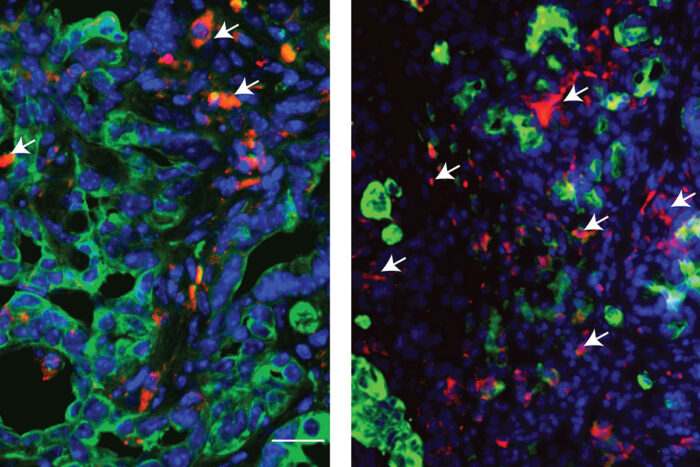 Patrick Grierson, Lin Li
Patrick Grierson, Lin LiResearchers at Washington University School of Medicine in St. Louis have identified a drug compound that makes pancreatic cancer cells more vulnerable to chemotherapy. While chemotherapy alone (left panel) can cause some degree of pancreatic cancer cell death, the addition of the compound ATI-450 enhances the effect of chemotherapy (right panel), leading to extensive cell death and disruption of the tumor architecture. Pancreatic cancer cells are shown in green. Dying cells are shown in red. White arrows also highlight dying cells.
Pancreatic cancer is extremely difficult to treat. By the time it is detected, the cancer often has reached an advanced stage, and patients usually do not survive longer than one year after diagnosis. An aggressive chemotherapy regimen is the first-line treatment, but the side effects can be severe, and many tumors stop responding to treatment.
Now, researchers at Washington University School of Medicine in St. Louis have identified a drug compound that makes pancreatic cancer cells more vulnerable to chemotherapy. Studying mice, they found evidence suggesting that the drug also may reduce some of the damaging side effects of the chemotherapy cocktail FOLFIRINOX (a combination of folinic acid, 5-fluorouracil, irinotecan and oxaliplatin), commonly used to treat pancreatic cancer.
The study is published Dec. 1 in the journal Science Translational Medicine.
“There is a tremendous need for new and better therapies for pancreatic cancer,” said senior author and medical oncologist Kian-Huat Lim, MD, PhD, an associate professor of medicine. “The drugs that we use now are powerful but often cause severe side effects, making it impossible to add more chemotherapy. This new drug appears to weaken the cancer cells and make them more sensitive to this particular chemotherapy regimen. In fact, the mice treated with chemotherapy plus this drug appear to be healthier than mice receiving chemotherapy alone, so there’s a chance the new drug is mitigating side effects.”
The drug, ATI-450, is an anti-inflammatory therapy and also is being investigated in clinical trials as a treatment for rheumatoid arthritis.
FOLFIRINOX is the front-line treatment for pancreatic cancer, but only about one in three patients will have tumor shrinkage following this therapy. Even then, this response only lasts six to seven months. Side effects are common and include nausea, vomiting, diarrhea, fatigue, hair loss, low blood counts and poor appetite.
As part of their research, the researchers found that a molecule called MK2 is critical in allowing the pancreatic tumor cells to survive chemotherapy. The molecule is highly activated in pancreatic cancer cells, where it turns on signaling pathways that favor survival and dial down cell death. ATI-450 is an attractive drug to test against these cancer cells because it is an MK2 inhibitor.
“MK2 turns on pro-survival mechanisms that allow cancer cells to adapt to the severe stresses of chemotherapy,” said first author and medical oncologist Patrick M. Grierson, MD, PhD, an assistant professor of medicine. “It also inhibits a type of cell death called apoptosis, thereby preventing tumor cells from dying in the face of chemo. This new drug inhibits MK2, so when the mice receive the drug at the same time that they receive chemotherapy, the tumor cells are much more susceptible to dying.”
Lim and Grierson, who both treat pancreatic cancer patients at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, and their colleagues tested the drug’s effects in pancreatic cancer cell lines growing in the lab, in mice harboring human pancreatic cancer cells, and in mice that developed pancreatic cancer naturally because they are genetically prone to the disease.
Compared with chemotherapy alone, the combination of ATI-450 plus chemotherapy reduced tumor size by about half. The mice receiving the combination treatment also survived longer, living an average of 41 days after the start of treatment compared with an average of 28 days for the mice receiving chemotherapy alone.
“A nearly 50% increase in survival is a significant improvement,” Grierson said. “On top of that, it’s impressive that the drug did not worsen side effects. There is a concern with chemotherapy for pancreatic cancer that incorporation of additional drugs will make the treatment even less tolerable. We monitored blood counts and assessed toxicities on the small intestine and colon, where we often see limiting toxicities, and the adverse effects were no worse with the combination treatment.”
Added Lim: “A lot of the side effects of chemotherapy are due to inflammation caused by molecules called cytokines. A benchmark for success in treating rheumatoid arthritis, which this drug was originally developed for, is the reduction of those cytokines. It’s possible we’re seeing the same effect in these mice.”
The researchers plan to continue investigating ATI-450 for pancreatic cancer, including whether this drug plus chemotherapy also can be combined with additional investigational treatments, such as immunotherapies. The researchers also said the drug has the potential to improve treatment for other cancers that use similar chemotherapy regimens, including colon and other gastrointestinal cancers.






