Drug development for severe respiratory diseases supported with $3.9 million grant
New drug candidate targets asthma, COPD, other progressive lung diseases
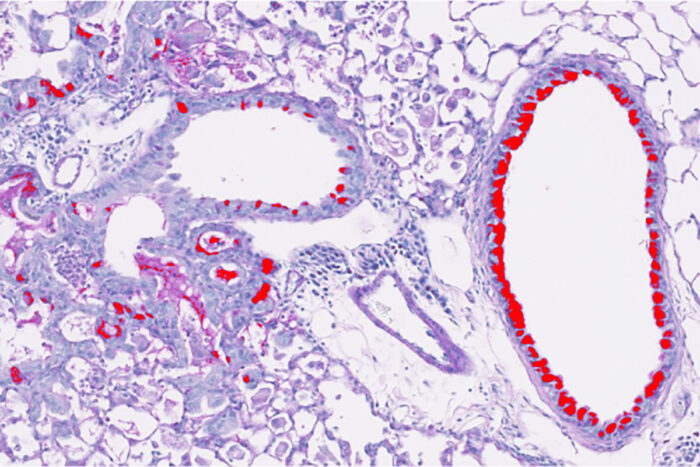 Kangyun Wu
Kangyun WuShown is a section of mouse lung with progressive lung disease, similar to asthma or COPD. The red staining shows excess mucus production that is characteristic of progressive lung disease and can occur in the aftermath of viral infection or other injury to the lungs. Researchers at Washington University School of Medicine in St. Louis have received a grant to develop a drug to block this process, potentially leading to a new treatment for debilitating lung disorders.
Researchers at Washington University School of Medicine in St. Louis have received a $3.9 million development award supporting new technologies and therapeutics to advance a first-in-class drug to treat debilitating lung diseases, including asthma and chronic obstructive pulmonary disease (COPD).
The research, funded by the Department of Defense, is led by Michael J. Holtzman, MD, the Selma and Herman Seldin Professor of Medicine and director of the Division of Pulmonary & Critical Care Medicine at Washington University.
Holtzman’s team has designed a new drug candidate that blocks two important signaling systems in the lungs. These systems cause cells of the respiratory tract to become mucus-producing cells. An overabundance of these cells creates too much mucus, which can block breathing. Evidence suggests that the drug could stop — and perhaps even reverse — progressive damage from overproduction of mucus that is triggered by aggravations to the lung, including from respiratory viruses, smoking and air pollution.
“Obstructive lung diseases, such as asthma and COPD, are the third leading cause of death due to disease in the United States,” Holtzman said. “But we have no effective treatments that address the root causes of these illnesses or halt disease progression. We can only try to relieve symptoms. This grant will allow us to continue research into a new drug candidate that our group developed and that has shown evidence of stopping and correcting what goes wrong in the lungs when this type of disease process is triggered.”
In past research, Holtzman and his colleagues identified specialized stem cells in the lungs that give rise to mucus cells. During development, such stem cells are responsible for growing the lung itself. Some of these stem cells remain in the lungs into adulthood and periodically make new cells to line the inner surfaces of lung tissue, including those cells that make mucus. These stem cells also respond to injury to the lung — such as a burn injury from inhaling smoke or a severe viral infection — and orchestrate the repair process.
 Kangyun Wu
Kangyun Wu“We need a certain level of mucus to protect the lungs from viruses or particles that can be inhaled,” Holtzman said. “The stem cell population is important in maintaining normal lung function and in injury situations, where they help with the healing process. But, unfortunately, in some people these cells can go off the rails. Under certain conditions, particularly a severe respiratory viral infection, these stem cells become reprogrammed so that they’re activated even after the injury or infection is resolved. This leads to overproduction of mucus and excessive inflammation that can interfere with lung function with airway obstruction and difficulty breathing.”
The new drug candidate is being designed to be taken by mouth or inhalation and to specifically target two related but distinct signaling molecules known as MAP kinases to control both arms of the immune and inflammatory response. Studying human cells, as well as mouse and pig models of respiratory disease following respiratory viral infections, the researchers found that not only does the drug reduce mucus production, it also nudges the rogue stem cells back toward their quieter and healthier state of readiness. The new grant will support studies in human cells, mice and pigs to establish evidence for the safety and effectiveness of the lead drug candidate and to help determine proper doses for subsequent studies in people. The safety work also will be facilitated by a subcontract to the team’s biotechnology company known as NuPeak Therapeutics, which is specially designed for this drug development activity. The goal is to gather data to support approval for a first-in-human clinical trial.
Holtzman said the drug does not treat the viral infection itself; rather, it stops what he calls post-viral disease and its progression, which includes asthma and COPD. Post-viral lung disease also could include COVID-19, as an example of another severe respiratory viral infection that causes progressive and in some cases long-term lung disease in some patients well after the infectious virus has been cleared.
SARS-CoV-2, the virus that causes COVID-19, is similar to the viruses Holtzman and colleagues are working with to study their new drug candidate. The group is gathering key information in patient and animal models to determine whether the same therapeutic strategy could prove useful in treating COVID-19 as it has been for lung disease due to other related respiratory viruses.
“We’re just beginning to learn how the body responds to SARS-CoV-2, but it is very common for any respiratory viral infection, especially severe infections, to trigger this progressive disease process in some percentage of the patients who contract the virus,” Holtzman said. “What’s interesting is that the infectious form of the virus is gone when this process ramps up. Patients aren’t fighting the virus any more, they’re fighting their own immune system. In future work, we will be interested in finding out whether our drug candidate can help shut this process down regardless of the trigger, viral or otherwise.”






