Implant provides lasting relief for treatment-resistant depression
Study finds enduring benefits of vagus nerve stimulation for patients not helped by standard therapies
 Sara Moser/WashU Medicine
Sara Moser/WashU MedicineIn a new study, WashU Medicine researchers show that vagus nerve stimulation from a small, implanted device provides substantial, long-lasting improvements for some participants with the most severe treatment-resistant depression.
About 20% of U.S. adults experience major depression in their lifetime. For most people, symptoms improve within a few treatment attempts, but up to one-third of patients have treatment-resistant depression, for which standard antidepressant medication or psychotherapy isn’t enough. Now, a study shows that a small, implanted device may provide substantial, long-lasting relief to people with the most severe treatment-resistant depression.
Researchers at Washington University School of Medicine in St. Louis supervised the large, multicenter clinical trial and found that the device, which stimulates the vagus nerve, produced improvements in depressive symptoms, quality of life and other measures, such as function, that were sustained for at least two years in the vast majority of patients who reported benefits after one year. On average, each patient had already tried 13 treatments that failed to help them, including interventions such as electroconvulsive therapy and transcranial magnetic stimulation, and had experienced depression for 29 years.
These new findings, reported from the ongoing RECOVER trial, were published Jan. 13 in the International Journal of Neuropsychopharmacology.
“We believe the sample in this trial represents the sickest treatment-resistant depressed patient sample ever studied in a clinical trial,” said lead author Charles Conway, MD, a professor of psychiatry and director of the WashU Medicine Treatment Resistant Mood Disorders Center. “There is a dire need to find effective treatments for these patients, who often have no other options. With this kind of chronic, disabling illness, even a partial response to treatment is life-altering, and with vagus nerve stimulation we’re seeing that benefit is lasting.”
Getting better, staying better
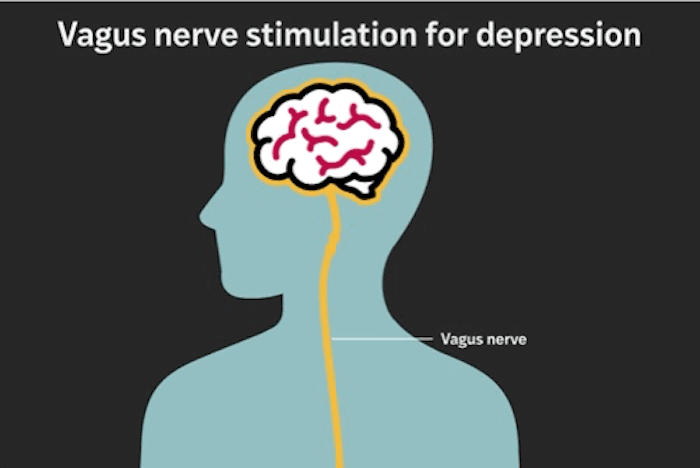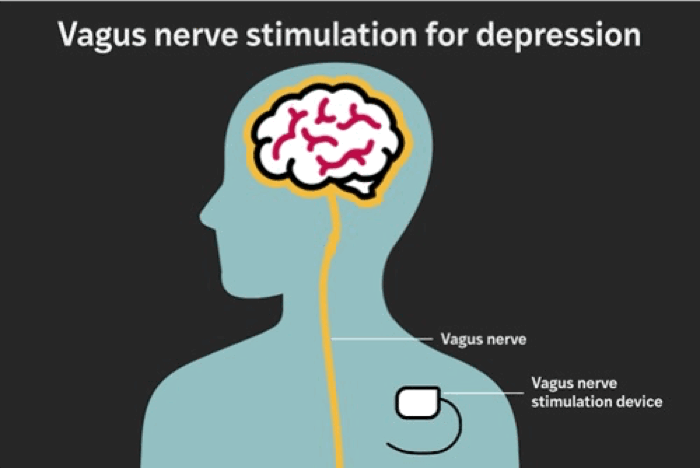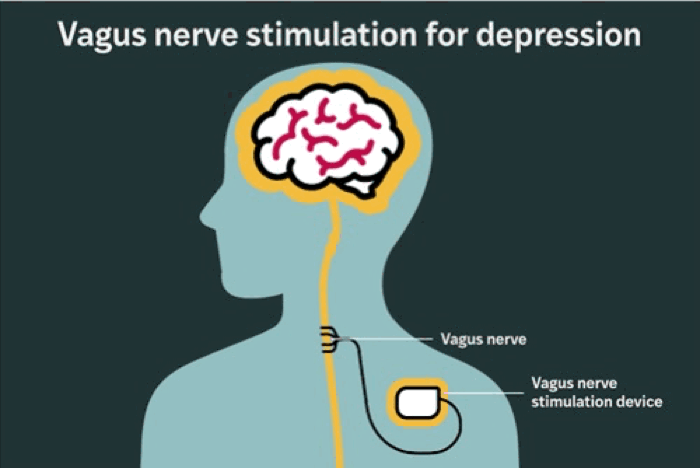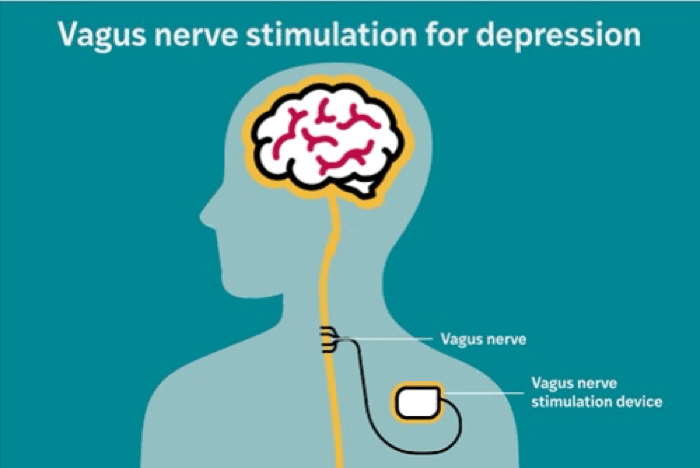
The RECOVER study was designed to evaluate whether adding vagus nerve stimulation (VNS) to existing treatment improves outcomes for treatment-resistant depression. The therapy involves implanting a device under the skin in the chest that emits carefully calibrated electrical pulses to the left vagus nerve — a major conduit between the brain and internal organs.
The device, the VNS Therapy System, is manufactured by LivaNova USA, Inc., which sponsored and funded the RECOVER trial. The trial aims to gather data on mood, function and quality of life outcomes for markedly treatment-resistant depression. The goal is that the U.S. Centers for Medicare and Medicaid Services (CMS) will use these data to determine future coverage of the therapy, which currently is out of reach to many patients because of its cost. Many private health insurance companies follow the lead of CMS when it comes to coverage, so a decision by CMS to cover the device and the surgery to implant it could make the treatment available to many more people.
The trial enrolled nearly 500 patients at 84 sites across the U.S. Three-quarters of the participants were so ill they were unable to work. VNS devices were implanted in each patient, but for study control purposes, only half of the devices were turned on during the first year of the trial. Treatment outcomes were measured in terms of depression symptom severity, quality of life and daily function.
Benefits for depressive symptoms were considered meaningful if a patient experienced at least a 30% reduction in symptoms from baseline, and “substantial” if symptoms declined by at least half.
Conway, who is the principal investigator of RECOVER, noted that even a 30% improvement can be life-changing for someone with severe depression, which can render people “paralyzed by life,” unable to accomplish the tasks of daily living and at significantly higher risk of hospitalization or early death.
Earlier reported outcomes from the blinded first year of the RECOVER trial demonstrated that people with activated devices spent significantly more time with improved depressive symptoms, quality of life and daily function than did those with inactive devices, although the primary outcome measure (the Montgomery-Åsberg depression scale, which measures the severity of depressive episodes) did not significantly differ between the two groups.
In this updated analysis of the ongoing trial, Conway and his colleagues examined only the patients who received active treatment during the first year of the RECOVER trial to determine whether improvements seen at 12 months would be sustained through 24 months. The team also assessed whether patients who showed no benefit at 12 months of activation might improve after more time with VNS.
Of 214 patients receiving active treatment from the beginning of the trial, about 69%, or 147 people, had a meaningful response at 12 months in at least one metric. Among those patients who experienced a meaningful benefit at 12 months, more than 80% maintained or increased benefits at 24 months across all measures of depression, quality of life and function. And among patients who had a substantial response at one year — defined as 50% or greater symptom reduction — 92% were typically still experiencing a benefit at the two-year mark, across all measures.
What’s more, nearly one-third of participants who had not responded after one year of treatment reported benefits at the end of the second year, suggesting the treatment might take more time to work in some people.
Among those who experienced benefits at one year, relapse rates were consistently low, especially for the strongest responders.
The researchers also found that more than 20% of treated participants, or 39 people, were in remission after 24 months — meaning their symptoms had improved to the point where they could function normally in daily life — a finding Conway said was particularly striking.
“We were shocked that one in five patients was effectively without depressive symptoms at the end of two years,” he said. “Seeing results like that for this complicated illness makes me optimistic about the future of this treatment. These results are highly atypical, as most studies of markedly treatment-resistant depression have very poor sustainability of benefit, certainly not at two years. We’re seeing people getting better and staying better.”






