Gene linked to metabolism drives deadly brain cancer
Could lead to targeted treatments for this aggressive disease
 Amit Gujar and Albert H. Kim
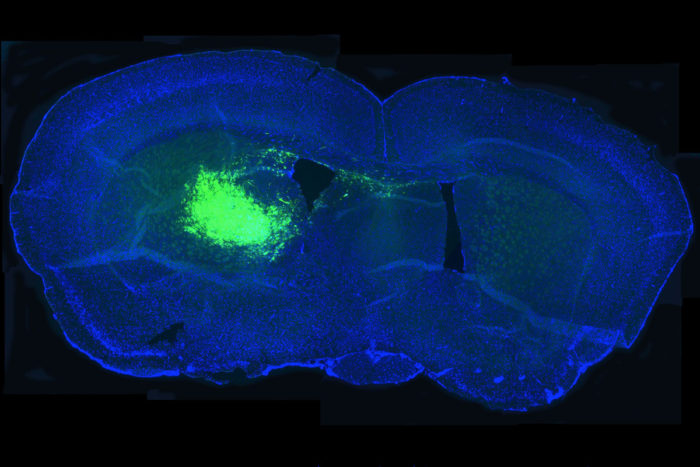
Amit Gujar and Albert H. KimResearchers have shown that a metabolic pathway associated with slowing aging also drives brain cancer. In the image above, cancer stem cells in a mouse brain section glow fluorescent green, allowing researchers to study the effect of inhibiting the pathway on the ability of cancer stem cells to survive and proliferate.
Scientists have identified a gene involved in cell metabolism and energy production that is overactive in a deadly form of brain cancer known as glioblastoma, according to a study at Washington University School of Medicine in St. Louis. The findings suggest that inhibiting that gene may improve the outlook for glioblastoma patients.
Glioblastoma is the most common and aggressive brain cancer in adults. Over 70 percent of patients with glioblastoma die within two years of diagnosis.
The new research showed that glioblastoma patients with high expression of a gene known as NAMPT died sooner. Tumors with elevated expression of the same gene grew rapidly when they were implanted in mice and shrank when NAMPT was inhibited.
NAMPT is a key component of a metabolic pathway known as the nicotinamide adenine dinucleotide (NAD+) pathway that is involved in producing cellular energy and plays a key role in several biological processes that depend on energy generation, such as aging, diabetes and inflammation.
The study is published Dec. 7 in Proceedings of the National Academy of Sciences.
Using human glioblastoma cells, Albert H. Kim, MD, PhD, an assistant professor of neurological surgery, postdoctoral researcher Amit Gujar, PhD, and colleagues showed that NAMPT helped cancerous stem cells survive and proliferate, fueling the growth of existing tumors, while inhibiting NAMPT reduced the ability of the cancer stem cells to renew themselves.
Furthermore, the scientists found that glioblastoma cells responded to radiation therapy – a standard therapy used to treat the disease in people – by increasing expression of NAD+ pathway genes, and that inhibiting NAMPT before dosing the cells with radiation made them easier to kill.
“If you target the NAD+ pathway, you can disrupt the ability of the cancer stem cells to self-renew, and you can also make them more sensitive to radiation treatment,” said Kim, who also treats patients with brain tumors at Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital. “In a patient, that could mean that if you suppress the pathway, the same dose of radiation may be more effective at destroying the tumor.”
The NAD+ pathway has recently attracted scientific attention because of its role in aging. NAMPT produces a molecule known as nicotinamide mononucleotide (NMN) which other scientists have shown to reduce signs of aging in mice. There is no evidence that NAMPT increases the incidence of cancer, even after prolonged administration. While its safety in people has yet to be determined – a clinical trial is ongoing in Japan – NMN and other molecules along the NAD+ pathway are being marketed as anti-aging supplements.
“There’s a lot of buzz about taking NAD+ precursors for their anti-aging effects, which is based on a lot of great science,” Kim said. “I don’t know if taking NAD+ precursors makes existing tumors grow faster, but one implication of our work is that we don’t yet fully understand all of the consequences of enhancing NAD+ levels.”
The NAD+ pathway involves many different genes and proteins, and its very complexity may be the key to having it both ways. Kim believes it may be possible to carefully modulate the pathway so as to suppress cancer without interfering with other important biological processes.
“The question we are considering now is, ‘How do we make an NAD+ strategy that is specific for cancer?’” Kim said. “Maybe there are some cancer-specific regulators, and we can disrupt those. Maybe we can change the expression of some key NAD+ pathway genes only in cancer cells, not healthy cells. There are many ways to look at this, and that’s why we want to dig deeper into how this pathway works in glioblastoma.”






