Novel approach shows promise against UTIs
Small molecules block bacteria from attaching to, infecting bladder
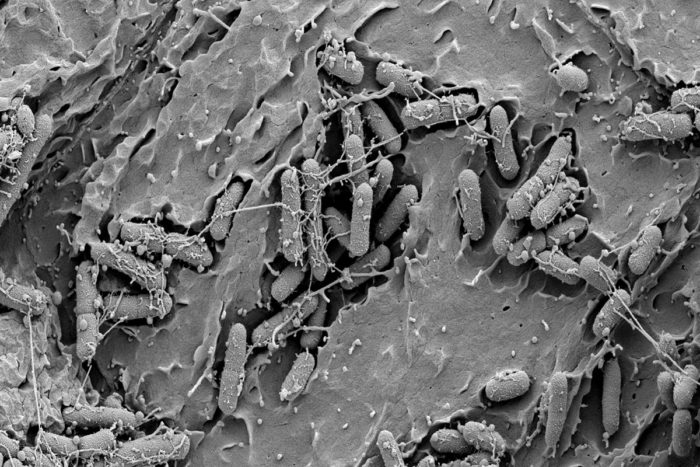 S. Hultgren, V. O'Brien, M. Joens, J. Fitzpatrick
S. Hultgren, V. O'Brien, M. Joens, J. FitzpatrickThe image shows the bladder of a mouse infected with E. coli. New compounds called C-mannosides have been shown to prevent these bacteria from attaching to the bladder wall, a potentially novel way to fight urinary tract infections.
Scientists at Washington University School of Medicine in St. Louis have designed small molecules that prevent bacteria from sticking to the wall of the bladder, halting the development of urinary tract infections (UTIs) in mice. Since the new compounds do not destroy microbes or block their replication — as traditional antibiotics do — the researchers anticipate that bacteria responsible for most UTIs would have a harder time evolving resistance to them.
The technology, a collaboration between James W. Janetka, PhD, and Scott J. Hultgren, PhD, is the basis of a startup company: Fimbrion Therapeutics. The novel approach to fighting UTIs recently has drawn the attention of GlaxoSmithKline (GSK), a global pharmaceutical company that is interested in exploring whether the compounds could be a viable treatment for UTIs in people. Fimbrion and GSK have formed a partnership to complete the preclinical optimization and development of these new drugs for UTIs.
Traditional antibiotics have long been effective treatment for UTIs. But microbes that have evolved clever defenses against these drugs are on the rise, creating a need for therapies that thwart bacteria in new ways, preferably without driving resistance.
“With increasing rates of antibiotic resistance, we are interested in finding new strategies to treat bacterial infections,” said Janetka, an associate professor of biochemistry and molecular biophysics. “The compounds we developed are promising because they prevent disease-causing bacteria from gaining a foothold in the bladder. They do not destroy microbes, so there should be no pressure favoring the survival of bacteria that are resistant to the compounds. We also have evidence that this may help preserve the beneficial bacterial communities already present in the microbiome.”
The newly developed compounds are called C-mannosides, and Janetka and his colleagues have shown that they can be delivered to mice by mouth in a liquid formulation to both treat existing UTIs and prevent such infections from developing. The C-mannosides bind with high affinity to a protein called FimH, a virulence factor that resides on the surface of uropathogenic E. coli (UPEC), preventing bacteria from docking there and infecting those cells. With nothing anchoring them in place, these bacteria pass naturally from the mice when they urinate.
The C-mannosides improve on the design of the researchers’ previously reported molecules called O-mannosides, which block bacterial attachment in the same way but were shown to be less stable to metabolism when given to mice. Research has shown that C-mannosides — linked with carbon atoms — appeared to maintain their structural integrity better than O-mannosides — linked with oxygen — as they passed through the mouse digestive and urinary tracts.
Working with Washington University’s Office of Technology Management, Janetka and Hultgren, the Helen L. Stoever Professor of Molecular Microbiology and director of the Center for Women’s Infectious Disease Research, licensed the technology to form Fimbrion Therapeutics and to develop mannosides and other drugs commercially that fight bacterial infections in novel ways that spare the use of traditional antibiotics.
“Fimbrion is a superb example of Washington University’s efforts to take promising laboratory findings and help nurture them into entrepreneurial endeavors,” said Provost Holden Thorp. “This is especially vital in developing new therapies that may help slow the ever increasing problem of antibiotic resistance. Fimbrion is filling a void in drug development aimed at stopping bacterial infections, and the partnership with GSK is an important step toward creating a new class of drug that would both treat common UTIs and reduce the spread of bacteria resistant to broad-spectrum antibiotics.”
The mission of Fimbrion is to design drugs that target the tactics bacteria use to establish infections, rather than their strategies for survival and replication. This minimizes the collateral damage of broad-spectrum antibiotics that may clear the infection but also deplete the body’s resident beneficial microbes and increase the spread of bacteria capable of surviving the treatment.
The collaboration with GSK is intended to help bring these compounds from the lab to the clinic. Before phase 1 clinical trials evaluating the safety of the drug can begin in people, the researchers first must select the best of the molecules they are currently testing in mouse animal models. They will focus on the compounds that are shown to be the most effective in preventing and treating UTIs and that are nontoxic in mice.
The investigators pointed out that the earliest stages of the technology were supported by a National Institutes of Health (NIH) Challenge Grant, as part of the American Recovery and Reinvestment Act of 2009. Moving the compounds down the drug discovery pipeline, the company co-founders said Fimbrion received support from Washington University’s Bear Cub program, which provides funding for promising translational research. This made it possible for the company to receive support from the NIH’s Small Business Innovation Research fund, aimed at helping bring promising biomedical technologies to the commercial marketplace.






