Patient defies genetic fate to avoid Alzheimer’s
Study offers clues to treatment, prevention
 Megan Farmer
Megan FarmerDoug Whitney, who lives near Seattle, Wash., inherited a mutation that has caused many family members to develop Alzheimer's disease at about age 50, yet he shows no sign of the illness at age 75. His case is the subject of a new study by WashU Medicine researchers that aims to identify potential routes to preventing or treating Alzheimer's based on Whitney's exceptional resilience to the disease.
Remarkably, Doug Whitney, 75, has escaped genetic destiny. Like many members of his family, Whitney inherited a rare genetic mutation that all but guarantees he would develop early-onset Alzheimer’s disease. But Whitney, whose relatives first showed symptoms of cognitive decline in their early 50s, remains mentally sharp with no signs of the devastating disease, and a new study by researchers at Washington University School of Medicine in St. Louis helps explain why.
Their findings, published Feb. 10, 2025, in Nature Medicine, point to potential therapeutic avenues to explore to help protect against the development of Alzheimer’s.
Combing through data on Whitney’s genes, and information gleaned from his brain scans and other biological features, the researchers identified changes in genes and proteins suggestive of resisting neurodegeneration. They also observed a near absence of the buildup in the brain of the key Alzheimer’s protein – tau – that is associated with the onset of disease symptoms such as cognitive decline.
“These extensive studies indicate a remarkable resistance to tau pathology and neurodegeneration,” said Randall J. Bateman, MD, the Charles F. and Joanne Knight Distinguished Professor of Neurology at WashU Medicine and co-senior author of the study.
 Megan Farmer
Megan FarmerWhitney, who lives near Seattle, Wash., was first identified as an exceptional case in 2011, when he arrived at WashU Medicine to enroll in a groundbreaking Alzheimer’s study: the Dominantly Inherited Alzheimer Network (DIAN) study. The international network focuses on families with inherited forms of Alzheimer’s disease that lead to cognitive decline at an early age, typically in a person’s 30s, 40s or 50s, and is led by WashU Medicine clinicians and researchers in the Charles F. and Joanne Knight Alzheimer Disease Research Center.
“When he came to WashU Medicine for the first time, together with his cousin, he was already 10 years past the age of onset for his family,” said Jorge Llibre-Guerra, MD, an assistant professor of neurology and co-first author of the study. Whitney’s family members with the same genetic variant typically showed signs of the onset of Alzheimer’s around 50 years of age. Because Whitney showed no sign of cognitive decline, WashU Medicine clinicians and researchers initially assumed that he was not a carrier of the genetic variant in the presenilin 2 (PSEN2) gene that had led to early-onset Alzheimer’s for every other member of his family who had inherited the genetic mutation.
“It came as a big surprise to learn that he was actually a mutation carrier,” said Llibre-Guerra. “At that point, he earned the title of the DIAN escapee, because he actually was able to escape the expected course of the disease.”
Whitney is one of three known escapees – more formally called “exceptional resilience mutation carriers” – in the world. The inherited form of Alzheimer’s that they have avoided accounts for fewer than 1% of all cases worldwide, and researchers expect that identifying successful treatments for this rare form of Alzheimer’s will inform prevention and treatment efforts for more common forms of the disease that develop later in life. So, identifying a case so rare and relatively early in a person’s life was an incredible opportunity for the WashU Medicine team: some unknown factor in Whitney’s body or in his genes was preventing the PSEN2 mutation from fully taking hold.
“If we are able uncover the mechanism behind this resilience,” said Llibre-Guerra, “we could try to replicate it with a targeted therapy designed to delay or prevent the onset of Alzheimer’s, leveraging the same protective factors that have kept Mr. Whitney from developing this disease to benefit others.”
Whatever that factor may be, Whitney is grateful to have the opportunity to contribute to the search for an effective treatment for the disease that has haunted his relatives for generations.
“My family has been devastated by this disease since the early 1900s, and probably going back further than that,” said Whitney. “It’s really important to me to figure this out. My mom had 13 brothers and sisters, and 10 died before they were 60 years old. It’s been a plague.”
The PSEN2 mutation Whitney carries is linked to an over-production of amyloid protein, which accumulates in the brain as the first stage in the progression of Alzheimer’s. The second stage, associated with the cognitive decline characteristic of the disease, involves a buildup of tau protein in the brain.
In this study, scans of Whitney’s brain showed a significant accumulation of amyloid but only a localized concentration of tau in his left occipital lobe. Tau usually spreads to multiple regions of the brain in typical inherited Alzheimer’s cases, with symptom onset associated with its presence in the medial temporal lobe, which is an area of the brain closely involved in memory.
“The goal now is to discover the precise reason why Mr. Whitney has escaped genetic fate,” Bateman said. “This discovery could become a powerful prevention for everyone. We call on researchers to help in the search.”
There are few clues as to what underlies Whitney’s resistance to tau accumulation. While the team identified that he carried specific genes that may be linked to staving off Alzheimer’s disease, the researchers do not yet have information on the genes’ functions. They also analyzed Whitney’s cerebrospinal fluid for biomolecules that might indicate unusual cellular activity in his brain and found a significantly higher-than-normal level of what are known as “heat shock” proteins.
Whitney worked as a shipboard mechanic for many years, enduring high temperatures in the engine rooms of naval vessels. That experience may have left him with elevated levels of heat shock proteins, which are created by the body to refold and repair other essential proteins in the body that have been damaged by stresses such as high temperatures.
“We don’t yet understand how or if heat shock proteins may be mediating the effect,” Llibre-Guerra said, acknowledging the established role of misfolded proteins in Alzheimer’s pathology. “However, in this case they may be involved in preventing aggregation and misfolding of tau proteins, but we do not know for sure.”
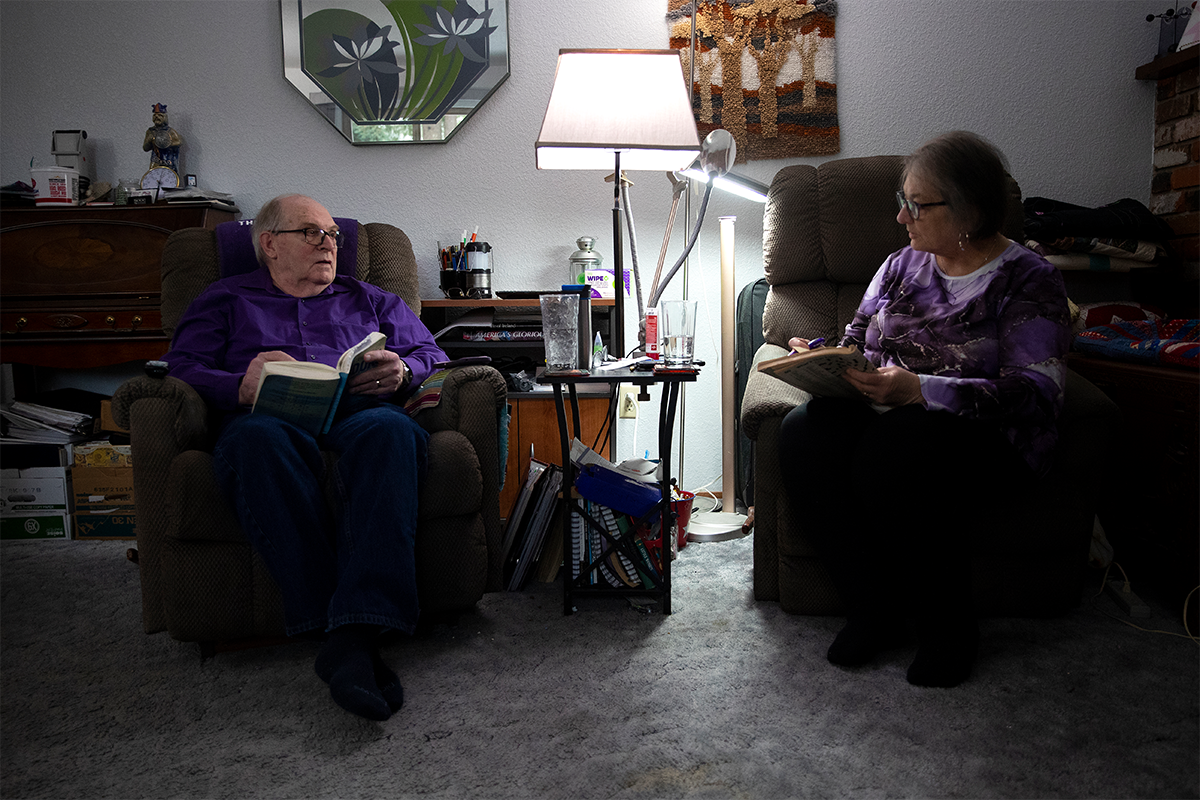 Megan Farmer
Megan FarmerLlibre-Guerra said he hopes the insights learned from Whitney will inspire future population studies or animal models to continue investigating this exceptional case.
“We have made all of the data we have available, as well as the tissue samples,” he said. “If researchers want to request those to do additional analysis, that’s something we would welcome.”






