Podcast: The making of a fast, accurate saliva test for COVID-19
This episode of 'Show Me the Science' focuses on a saliva test developed by School of Medicine scientists to detect the virus that causes COVID-19
 Matt Miller
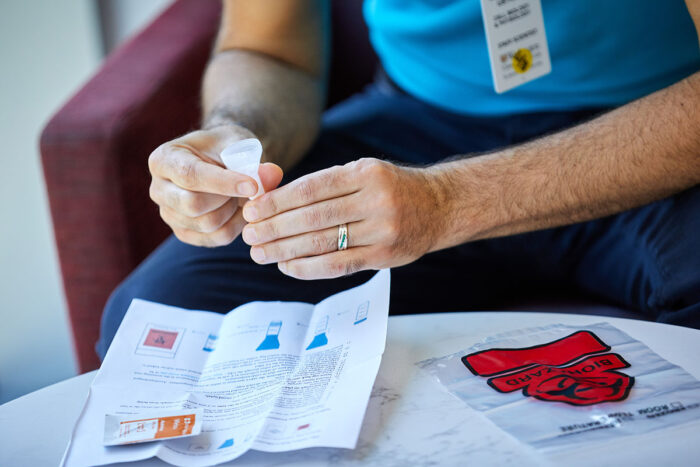
Matt MillerResearchers at Washington University School of Medicine in St. Louis have developed a saliva-based test for COVID-19 that is faster and easier than the swab tests currently in use. Recently, volunteers from the university took part in a study to evaluate the feasibility of the saliva test. The university also is using the new test to regularly test students.
A new episode of our podcast, “Show Me the Science,” has been posted. At present, these podcast episodes are highlighting research and patient care on the Washington University Medical Campus as our scientists and clinicians confront the COVID-19 pandemic.
Since the start of the COVID-19 pandemic, health officials have talked about the need for better, faster and more frequent testing. Recently, researchers at Washington University School of Medicine in St. Louis developed a saliva test that can detect the SARS-CoV-2 virus without inserting a nasopharyngeal swab into the nose or throat.
The saliva test also doesn’t require chemical reagents to extract RNA from the sample. Such reagents have been in short supply, often resulting in delays in reporting test results. The test can be run in a few hours and, ideally, can return results the next day. Further, it can test for more than one virus at a time, making it particularly useful as the COVID-19 outbreak stretches into flu season. The new test was developed by a team from the School of Medicine’s Department of Genetics and the McDonnell Genome Institute, in collaboration with the biotechnology company Fluidigm. The test has attracted the attention of state officials in Missouri, who are planning to use the test to screen populations known to be at risk for the virus.
 Related: Washington University develops COVID-19 saliva test
Related: Washington University develops COVID-19 saliva test
Test is faster, simpler than nasal, oral swab tests and enables screening on a massive scale
In this episode, scientists Jeffrey Milbrandt, MD, PhD, and Richard Head discuss why they believe the saliva test will be important in detecting the virus’s presence even before people begin having symptoms, including in individuals who remain asymptomatic. Milbrandt is the James S. McDonnell Professor and head of the Department of Genetics and the McDonnell Genome Institute, and Head is a professor of genetics and director of the Genome Technology Access Center at the McDonnell Genome Institute.
The podcast, “Show Me the Science,” is produced by the Office of Medical Public Affairs at Washington University School of Medicine in St. Louis.
Transcript
[music]
Jim Dryden (host): Hello, and welcome to Show Me the Science, conversations about science and health with the people of Washington University School of Medicine in St. Louis, Missouri, the Show-Me State. As we continue to look at Washington University’s response to the COVID-19 pandemic, better testing is an area that many say is key to controlling the outbreak. And a major recent step in the Washington University response came when the US Food and Drug Administration granted emergency use authorization to a new saliva-based coronavirus test developed by researchers at the School of Medicine’s Department of Genetics and the McDonnell Genome Institute. Here in the Show-Me State, the governor and the first lady of Missouri recently were shown to test positive for the virus. But before he tested positive himself, the new saliva test brought Missouri governor Mike Parson to campus.
Mike Parson: By enabling rapid testing, this new saliva test is a major development that will improve our testing capabilities even more. I want to thank WashU and all the researchers, not only for their work on this new test, but also their continued support throughout the COVID-19 crisis.
Dryden: The saliva test is fast, accurate and inexpensive. One of the test developers, Jeffrey Milbrandt, also spoke to members of the press during the governor’s visit.
Jeffrey Milbrandt, MD, PhD: So the test requires only a small amount of saliva collected by spitting into this tube. It is very accurate. It is quick, simple, and is not limited by supply-chain shortages that we’ve seen in others. It’s also very economical. Importantly, this saliva test can be altered in the future to accommodate other viruses such as the influenza viruses that will soon raise their head.
Dryden: Milbrandt is the head of genetics and of the McDonnell Genome Institute. He says the test was designed to run on a machine that already was being used at the Genome Institute, one of the original centers that was involved in the mapping and sequencing of the human genome.
Milbrandt: We hope that we will always be able to give a result in about 24 hours.
Richard Head: I’m Richard Head. I’m a professor of genetics and I’m also the director of the Genome Technology Access Center within the McDonnell Genome Institute.
Dryden: Richard Head also worked to develop the saliva test. He says for months, there’s been an urgent need to simplify testing so that infected people can be identified easily and quickly. And the test does not require anyone to stick a long nasopharyngeal swab, or NP swab, up anyone else’s nose. In this episode, we discuss the new saliva test with Milbrandt and Head from Washington University’s McDonnell Genome Institute.
[music]
Dryden: Testing has been a problem since the beginning of the pandemic. How to test, who to test, how often to test. How does this new saliva test contribute to answering the need for faster, better testing? At least at Washington University and in our region, how does this fit into that question, I guess?
Head: I think there are really three ways. The first way is that the original tests that were being done, NP swabs, have met with a number of supply-chain issues as a result of maxing out the capacity for the reagents. And so as we continue to expand the need for testing, and let’s face it, this is really unprecedented in the amount of testing that we’re doing for anything and the amount of time that we’re doing it, so the supply chain that has existed for the tests that have been used has really been maxed out. And so in order to continue to expand, we really need additional mechanisms for testing that use different types of equipment, different types of reagents, etc., so that we’re able to continue to expand the testing need to fill that gap. I think another aspect of this is that anyone who’s ever had an NP swab or has seen an NP swab done, knows that they’re extremely uncomfortable. And so, even though that’s been the standard test, I don’t think anyone was ever hoping that would continue to always be the only option moving forward, to the point where a lot of folks have probably avoided getting a test, not only because of the availability of tests early on but because of the discomfort involved, so having a test like a saliva test that uses different types of equipment and reagents that’s also much more comfortable or there’s really no discomfort at all. We’ve also noted that there are some sampling issues with NP swabs that the saliva tests seem to be– because of the fact that you don’t have the discomfort factor, we’re able to detect certain individuals that NP swabs miss and vice versa. So I think having multiple avenues to test also gives us a better overall sampling and understanding of the prevalence of the virus in the community.
Dryden: Now this is not the only saliva test that has been approved for emergency use authorization, but this is a different type of test in that you don’t have to go through one of the key steps in many of the other tests, which is extracting RNA from the sample. Can you first tell me what RNA extraction is and why eliminating that step might be an advantage here?
Head: Sure. So the RNA-extraction step is something that’s common for the NP swabs and it’s also involved in some of the saliva assays. And what this is, is this is a step where you have to take the sample, and usually a significant volume of the sample, and you take it through a procedure that allows you to extract the RNA from the viral particles. And it requires kits or reagents that are necessary to do that step. Tests like ours does not require that extraction step. We actually take the crude saliva sample directly into the assay, so it saves on the need for those reagents which can also be limiting due to supply-chain issues, since many, many of the different tests require that step to occur.
Dryden: You guys developed this test with the idea of using a machine that you already had at the McDonnell Genome Center, correct?
Head: That’s correct. We use the Fluidigm Biomark platform for this, which we’ve been using here for both clinical and research testing for about eight years.
Dryden: What kind of testing? What is it normally used for?
Head: We use it for tests similar to the ones that we’re running now. We’ve used it for looking at disseminated tumor cells in bone marrow and it’s a fairly robust platform.
Dryden: Now, Dr. Milbrandt, I want to ask you a question. What was the harder thing to do here? To develop this test, or to make it possible to scale it up so that it could be used? I mean, to do things like coming up with a funnel to catch the saliva, getting it made, putting the kits together, setting up this whole infrastructure. Was the science part easier, or the organizational, how to actually implement it part?
Milbrandt: I think the science part is easier once you discover the answer. I mean, so, it was a broad-based team of people. That’s the key step here, the infrastructure that has been developed at the MGI over the years in terms of personnel and expertise and equipment. We have expertise on developing the kits, finding samples, learning how to take the specimens in the very best, most efficient way through occupational health, to help the student screening. All those attributes came together and organizing that was an interesting exercise. But I think that part now is complete and you see the results. There were a couple solutions with the saliva that adapted it, that Rich and his team came up with. Adapted it to the Fluidigm, which really set the stage for this rapid, non-supply chain limited, very sensitive saliva test.
Dryden: Now, for either of you, how long does it take to actually run a test? I participated a couple of weeks ago in one of the WashU studies and we got results back in 48 hours, but the test itself, is this something can be run in minutes? Does it take hours on the machine? How long does an individual sample take?
Head: From the time that we start to physically process a sample to the time a result comes off of the instrument and can be viewed in a report, is on the order of about three and a half to four hours.
Dryden: So it’s much faster, also, than the NP swab test?
Milbrandt: It can be, but I think it shouldn’t be characterized as the so-called rapid test. We strive for 12- to 24-hour turnaround for the test, so that would put it not in the category that people are talking now about rapid-turnaround tests. So it’s not one of those but it’s good turnaround time.
Dryden: Well, and I know it wasn’t just how long it took to run an actual test, but we were, earlier in the summer, having the experience of people waiting five, six, seven, eight days in order to get results. Even if the results were negative, eight days later you might be positive. I mean, it wasn’t turning around quickly enough to do much good, I guess.
Milbrandt: Yeah. It’s an important point that’s now being recognized that testing needs to be turned around probably within 24 hours to really make it useful for public health purposes. Rich mentioned that the NP swab, the swabs, actually, were limiting for a time. So you couldn’t perform the swab, the actual test. The other thing about the NP swab is that it also exposes the health-care worker. So you need a health-care worker, you need a nurse to actually do the NP swab. That takes time. That costs money. But also it exposes that worker to potential viral infection. Imagine if the person sneezes when you’re doing the test, so there are extra protective equipment that needs to be used for that purpose and saliva eliminates a number of those as well.
Dryden: We’re now heading into the fall. School has started again. This has long been predicted to be a time period where we might be experiencing a second wave. How does having a test like this, in the arsenal, make it possible now to perhaps better prevent or contain the pandemic in the months ahead, at least in this region where the test is available?
Milbrandt: If you can screen quickly and you can screen large numbers of people and you know where the infection lies, you can stop the spread with social distancing, quarantining, best practices for preventing the spread. But if you don’t, of course, know where the asymptomatic patients or even symptomatic patients are located, without testing then, of course, you can’t institute those measures. So the availability of a test that can do large numbers of people and pick up asymptomatic infections can be very helpful. The other thing that should be said about this test is that we can— in the fall, now, we’re worried about flu at the same time as COVID. How will you distinguish the symptoms? It will be a diagnostic nightmare in the beginning. And this test can easily be adapted to add additional viruses, so that when you run the diagnostic test for no extra time and little extra cost, we will be able to screen for multiple viruses at once as the Fluidigm Corporation comes out with those new chips. So we’re excited about that and I think that will be another big help in mitigating some of the issues we’re going to come up with in the fall.
Dryden: Is there a target on how many tests you want to run? Who you want to test? I mean, I know that when the freshmen showed up at their dorms at WashU, they were doing the saliva test. You were running that. But what about other schools or businesses in the region? How big are you looking, I guess?
Milbrandt: As you know, the state of Missouri, they’re very interested in exporting our test to other sites within the state of Missouri and disseminating the testing platform throughout the state of Missouri. We’ll be doing a large number of individuals for the state. The priority is to take care of the students, work with the state. There are so many institutions — colleges, public schools, businesses, assisted-care facilities —that need our help. We cannot help everyone that we would like to be able to help. We’re prioritizing that list and trying to do the testing where we can make the biggest impact. One thing I would like to mention, that there are NIH grants that will likely be awarded now, to Washington University, that will help in the underserved populations.
Dryden: Rich, did you want to add anything to that?
Head: Just to follow up on what Jeff said. Yeah. We’ve been working with and communicating with a number of other facilities, both in the state of Missouri and even some outside of Missouri as well, that have had interest in the platform and the test format. Basically, we’re trying to give them what we know about the tests and make it easier for them to make a decision as to whether or not the use of this particular platform would work in their environment as well. And basically, just disseminate the test as broadly as we can.
Dryden: Would you want to screen everybody, like happened with the freshmen when they were moving in, or do you want to test people who seem to be symptomatic, or— Who gets this test in the places where it might be available?
Milbrandt: We’re going to let the state determine somewhat what populations they think are best to screen. Certainly, the assisted-care facilities will be one of those that they’re very interested in doing, but much of this is flowing from the state in terms of the screening and surveillance that’s necessary.
Dryden: As we’re having this conversation today, a lot of us who work at WashU are there sometimes and other times we’re working from home. Would you see this as something that might be used, “Coming to work on Monday, it’s test day,” or how will that work?
Milbrandt: We did consider that possibility. As we ran a number of the pilots, we found that the percent positivity rate amongst our employees, as well as amongst many of the students, was very low. Much lower than we anticipated, so that the decision has been made now that repetitive testing of Washington University employees will probably not be necessary if things continue as they are now.
Dryden: And one final question. We always talk about this test as being inexpensive. How inexpensive is it? I mean, if you have a lot of money, $1,000 might not be all that expensive. But for me, $5 is a little bit more reasonable. I assume it’s somewhere in that range, but how inexpensive is it?
Milbrandt: You are correct, Jim. It is somewhere between five and a thousand dollars.
[laughter]
Dryden: Well, thank you.
Milbrandt: We haven’t established all of the guidelines for final pricing yet. There’s a number of factors that need to be considered, but we’re working on that diligently.
[music]
Dryden: As use of the new saliva test ramps up in the St. Louis region and in the state of Missouri, Milbrandt and Head believe they have the capacity to do 20 to 30,000 tests per week. They also have an agreement in place to get more machines from the Fluidigm Corporation in order to run even more tests. And, in theory, more testing — along with quarantining, wearing masks, and contact tracing — are key steps toward getting life back to something that’s a little closer to normal while we wait for more effective therapies and vaccines. Show Me the Science is a production of the Office of Medical Public Affairs at Washington University School of Medicine in St. Louis. The goal of this project is to keep you informed and maybe teach you some things that will give you hope. Thank you for tuning in. I’m Jim Dryden. Stay safe.
[music]






