Podcast: Wildlife surveillance may help identify the next pandemic
This episode of 'Show Me the Science' focuses on monitoring and identifying pathogens that might jump from animals into humans
 Ryan Peters
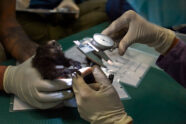
Ryan PetersScientists prepare to take a skin biopsy from the wing of a bat as part of a health check in Peru. Wildlife biologists, infectious disease experts and others, including scientists at Washington University School of Medicine in St. Louis, are proposing a decentralized, global wildlife biosurveillance system to identify — before the next pandemic emerges — animal viruses that have the potential to cause human disease.
A new episode of our podcast, “Show Me the Science,” has been posted. At present, these episodes are highlighting research and patient care on the Washington University Medical Campus as our scientists and clinicians confront the COVID-19 pandemic.
The virus that causes COVID-19 is thought to have originated in wild bats that live in caves around Wuhan, China. Many devastating epidemics in recent years — including SARS, Ebola and HIV/AIDS — were caused by animal viruses that spilled over into people. Before another pandemic begins, a diverse group of infectious disease experts, ecologists, wildlife biologists and other experts say that a new, decentralized, global system of wildlife surveillance must be established to identify animal viruses in wild animals that have the potential to infect and sicken people.
In this episode, Jennifer A. Philips, MD, PhD, an associate professor of medicine and co-director of the Division of Infectious Diseases at Washington University School of Medicine in St. Louis, and Gideon Erkenswick, PhD, a postdoctoral research associate in Philips’ lab and director of Field Projects International, discuss why we need an early-detection system for viruses that have the potential to trigger the next pandemic and how it would work.
The podcast “Show Me the Science” is produced by the Office of Medical Public Affairs at Washington University School of Medicine in St. Louis.
 Related: Global wildlife surveillance could provide early warning for next pandemic
Related: Global wildlife surveillance could provide early warning for next pandemic
Experts propose decentralized system to monitor wildlife markets, other hot spots
Transcript
[music plays]
Jim Dryden (host): Hello, and welcome to “Show Me the Science,” a podcast about the research, teaching and patient care as well as the students, staff and faculty at Washington University School of Medicine in St. Louis, Missouri, the Show Me state. My name is Jim Dryden, and I’m your host this week. We’ve been focusing these podcasts on the COVID-19 pandemic and Washington University’s response. This week, we’re discussing where COVID-19 might have come from and how to detect the next virus with pandemic potential before it spreads. It’s thought that the virus that causes COVID-19 may have originated in wild bats that live in caves around Wuhan, China. Like many devastating epidemics in recent decades from Ebola to HIV/AIDS, it’s thought this pandemic was triggered by an animal virus that spilled over into the human population. Now, a diverse group of infectious diseases experts, wildlife biologists, ecologists and others, including researchers at Washington University School of Medicine in St. Louis, is proposing that a global system of wildlife surveillance could – and should – be established to identify viruses from animals that have the potential to infect people.
Jennifer Philips, MD, PhD: It’s hard to say how often it happens, but it’s happened enough in our history, and when the wrong pathogen enters the human population where we don’t have adequate immunity, then the cost to human life can be enormous. And as we’ve seen the social disruption that is caused can also be really tremendous.
Dryden: That’s Washington University infectious diseases specialist Jennifer Philips, one of the authors of a new article on biosurveillance published in the journal Science. She’s part of the international team proposing that we pay closer attention to emerging infectious diseases by monitoring wild animals around the world. It would have been very difficult to do this sort of work even 10 years ago, but Gideon Erkenswick, a postdoctoral researcher in Philips’ laboratory, who also is a director of Field Projects International, a research organization focused on studying wildlife, says it is possible now to do complicated surveillance even in very remote places.
Gideon Erkenswick, PhD: There is and have been few groups that have been doing this work. There’s been a lot of hand-waving from individuals that have recognized for a long time how important biosurveillance is, but there’s not been a lot of commitment to it internationally. For example, we still don’t have any standardized international regulation on the movement of wildlife that reflects disease risks.
Dryden: Currently, there are scientists studying animals stationed in many places around the world. Erkenswick says with the technology now available, it would be possible to put resources together to learn more about potentially emerging viruses before they can cross from some of those animals into humans. He says a decentralized, global wildlife biosurveillance system would have the potential to identify viruses that could cause human disease before the next pandemic emerges.
Erkenswick: You would have diverse groups of people who are trained and cognizant of the risks we are now all very aware of, especially with SARS-CoV-2. You would be collecting samples from bats. You would visit caves, you would go into natural habitat, collect samples in wildlife markets. You probably would collect samples from rural communities where there’s a lot of overlap between wildlife habitats, and then you would bring them to a nearby location where, again, using safe technique you extract RNA and DNA and directly on-site sequence for conserved regions within viral genomes. You can prescreen things fairly rapidly for the presence of an infectious agent.
Dryden: So without getting too terribly technical, what kind of tools would those be?
Erkenswick: So the kind of things we’re talking about in terms of new technology are portable sequencers. There’s a new sequencer that’s literally the size of a USB stick and can fit in your pocket, and you can carry that to a rainforest, and then you sequence it right on the spot. If you do encounter something like SARS-CoV-2, if you chance upon it, do you really want to be collecting it, storing it, bringing it all over the place before you figure out if there’s something potentially worrisome in it? The ideal situation is, on the spot, you can safely assess whether there is something there.
Dryden: And it’s not as simple as looking for sick animals in this cave or in the wet market because the viruses that would affect us like SARS-CoV-2 may or may not make the animals sick, correct?
Philips: Right. That’s absolutely true. So especially bats are able to tolerate a lot of viruses without necessarily being sick. And so just sampling sick animals is not really an adequate way to do biosurveillance. There have been a small number of government-funded biosurveillance efforts. And these programs are really sort of groundbreaking in beginning to do this kind of work, and they have done amazing work, but they’re very focused and localized so the sampling is quite intermittent in just certain locations. And there’s just few regional labs that do the analysis. And so there hasn’t – that’s, I guess, the start of what’s needed, but it hasn’t really provided the kind of global sampling that is probably needed. Or touched upon the variety of environments in wildlife markets and in the wildlife trade, where humans and wildlife do come into contact with one another. And I think the other point that’s worth making is that these biosurveillance systems that we do have right now, because there’s sort of no global consensus, no global effort, they’re subject to political whims of a few countries.
Dryden: Now, how common is it that animal viruses can cross over into humans? It’s not uncommon, correct?
Philips: When it happens, we only know about it if there is really transmission between humans or ongoing transmission, or if we happen to identify an individual who’s sick and comes to medical attention and actually gets a rare diagnosis. So we don’t know how many times it necessarily happens, but we know it’s happened many times in our lifetime. So even in the last 20 years, this has happened at least three times in the case of coronaviruses, starting with the SARS epidemic and then MERS and now, of course, SARS-CoV-2, the virus that is causing the COVID-19 pandemic. And it’s not restricted to coronaviruses either, so other viruses in our lifetime have transmitted infection and those include HIV and Ebola among others. It’s hard to say how often it happens, but it’s happened enough in our history. And when the wrong pathogen enters the human population where we don’t have adequate immunity, then the cost to human life can be enormous. And as we’ve seen, the social disruption that is caused can also be really tremendous.
Dryden: So where does the idea for a decentralized, global surveillance system come from?
Erkenswick: There is and have been few groups that have been doing this work. And there’s been a lot of hand-waving from individuals that have recognized for a long time how important biosurveillance is, but there’s not been a lot of commitment to it internationally. For example, we still don’t have any standardized international regulation on the movement of wildlife that reflects disease risks.
Philips: Well, I think this came together because we really brought together a group of individuals who have really diverse expertise and interests, people with human, animal and environmental expertise, with the understanding that all of these things are really inextricably linked together. We thought that bringing this group of people together was important to try to come up with ideas of how we could prevent this kind of thing from happening again in the future. And, as a group, we brainstormed what could be critical next steps to create more effective biosurveillance infrastructure, I would say. That’s something that would have to be practical, affordable. It has to be safe for people who are doing the biosurveillance or the sampling. It has to be feasible. But one thing that’s really happened in the last five years is that there’s more affordable and portable technology that could really be implemented more widely around the world and could take advantage of the local expertise at, really, sites of wildlife-human interface.
Erkenswick: That’s why we did it. We felt there was a need for this diverse group of people’s voices to be heard in a collective way. You want to go to places where there’s no – there’s very little overlap between human communities and wildlife populations. So you know what’s natural because it is totally normal for a species to have their own viruses and bacterial infections, and they cope with them, and they survive just fine, and they’re not necessarily infectious to us. So you want to know what’s in the natural system, then you want to know what’s different when these animals, wildlife and humans are in contact.
Dryden: Now a system like this would do surveillance of wild animals like bats, certainly, but what about domestic animals, even pets?
Erkenswick: Domestic animals and pets are very much part of that human-wildlife interface. If you live in the rainforest in Southeast Asia and you have a dog or a cat, and it’s roaming around and goes into the forests and attacks a primate or a bat, so yeah, absolutely. And you want to know if that exchange is being mediated by domestic animals, absolutely.
Dryden: What sorts of global biosurveillance systems do we have in place now, if any?
Erkenswick: In the lead up to this article, some of the things we did was contact a lot of colleagues we know throughout the world that do wildlife research – in Madagascar, in Indonesia, in Peru, in Ecuador – and we just literally surveyed friends and associates, “Where do you take animals when they’re sick?” And in almost every situation, it was, “Nowhere.” We dispose of things. We collect things. We store them, but really there’s nobody offering to do this work, and it’s expensive. Here in the U.S., we have just vaulted forward on our capacity to do pathogen screening. I mean, in high school, you have kids running PCR machines and extracting DNA and in some cases sequencing DNA. In places where zoonotic risks are greatest, you just have light microscopes. It’s not that the capacity to do genetic or genomic-based pathogen detection couldn’t be there. There are smart people with a lot of experience that just don’t have the tools. And the idea behind this paper is that it can be done affordably. We can utilize all of those people on the ground to do much better biosurveillance.
Dryden: If they have the equipment in place, is it easy to know that this is something that can cross over from one species to another, or do you know that it can make people sick when people start getting sick?
Philips: There’s not one answer to that question. Part of risk assessment builds upon all the knowledge that our virologists have built up over the years. So for some microbes or some viruses that we know are related to viruses that infect humans like coronaviruses, then we start to learn something about that. And we can make some predictions. We know whether a population of viruses now that are circulating in bats have the sequence that would allow them to attach to the receptors that would enable them to infect human cells. Or we may know something about whether they’re dissimilar enough from circulating viruses that humans might lack immunity to those viruses. So sometimes it builds upon the knowledge that we have. And then sometimes we can take new viruses and see whether they do infect human cells in the lab, or even just whether if we replace the sequence of a virus that we work within the lab with a sequence that’s circulating in the wild, how would that impact its ability to infect human cells or to infect an animal model. And then I think there’s a lot of unknown about just what is circulating and what is the potential. And so part of it is starting to gain more information about that interface.
Dryden: So this is a way to identify viruses, but can knowing about these viruses keep us safer, maybe even prevent this type of outbreak next time?
Philips: Yeah. I think especially – and this is very much what happens with influenza. I think that’s the case where it’s most developed. And one can certainly see with coronaviruses that having this information allows you to stay ahead in terms of therapeutics and interventions. So hopefully, we will have effective therapeutics. We’ll have monoclonal antibodies, maybe, and vaccines that work for SARS-CoV-2. And we would like to have more broadly neutralizing antibodies or broadly effective therapeutics. And we can ensure that we stay ahead of what’s in the wildlife population, right, that interface. We’ve had three epidemics in 20 years. It’s not that rare an event. So we should be trying to make sure that we can stay ahead in terms of our therapies and our interventions.
[music plays]
Dryden: Erkenswick, Philips and their colleagues around the world have proposed better biosurveillance, but governments and funders still need to cooperate to make the idea a reality. But considering the thousands who have died and been hospitalized and the millions of jobs lost due to the pandemic, perhaps setting up such programs to screen for viruses would be a money saver in the long run. They say that as humans and animals continue to interact in new ways and in new locations, it’s virtually certain other animal pathogens also will cross over to humans in the future. But without a decentralized, global system of biosurveillance, it won’t be possible to predict how and when that will happen.
Show Me the Science is a production of the Office of Medical Public Affairs at Washington University School of Medicine in St. Louis. The goal of this project is to keep you informed and maybe teach you some things that will give you hope. Thanks for tuning in. I’m Jim Dryden. Stay safe.






