Scientists ID source of damaging inflammation after heart attack
Existing drug dials down inflammation in mice
 Lora Staloch
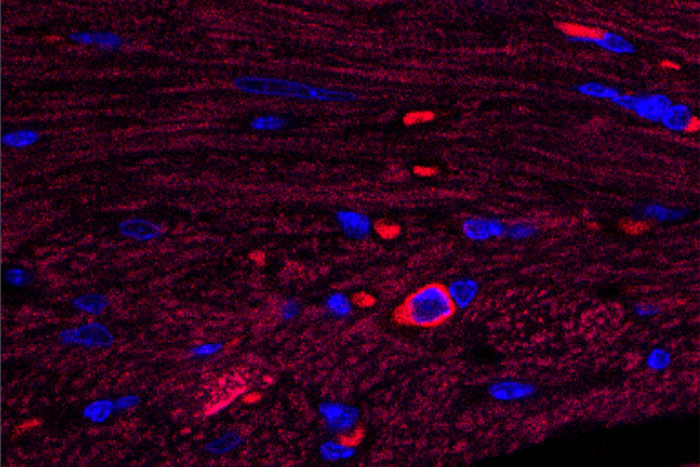
Lora StalochIn this image of heart tissue, a single cardiac B cell lymphocyte is visible (blue surrounded by bright red). This type of immune cell triggers damaging inflammation after a heart attack, according to researchers at Washington University School of Medicine in St. Louis. Their study also shows that the drug pirfenidone reduces this inflammatory response to injury and protects the heart from progressive damage.
Scientists have zeroed in on a culprit that spurs damaging inflammation in the heart following a heart attack. The guilty party is a type of immune cell that tries to heal the injured heart but instead triggers inflammation that leads to even more damage.
Further, the researchers have found that an already approved drug effectively tamps down such inflammation in mice, protecting the heart from the progressive damage that often occurs after a heart attack.
The study, from Washington University School of Medicine in St. Louis, is published June 7 in JCI Insight.
Led by senior author Douglas L. Mann, MD, director of the university’s Cardiovascular Division and cardiologist-in-chief at Barnes-Jewish Hospital, the study showed that mice modeling human heart attacks lived longer if then given pirfenidone, a drug already approved to treat an unrelated lung condition. Further, the research showed that the drug works by regulating in the heart the specific response of B cell lymphocytes, the immune cells that the scientists discovered were responsible for the inflammation.
“If we understand how pirfenidone works to reduce inflammation, we can work to modify the drug or make an even better drug that may be able to help a large number of patients,” said Mann, who is also the Tobias and Hortense Lewin Distinguished Professor of Cardiovascular Diseases.
In a heart attack, blood is cut off from an area of the heart that then often dies. If the person survives, the body tries to heal the dead muscle by forming scar tissue — but such tissue can further weaken the heart. Yet another wave of damage can occur when well-intentioned immune cells try to heal the injured heart but instead drive inflammation. Finding ways to prevent progressive inflammatory injury to the heart could help some 1.5 million heart attack patients in the U.S. annually, potentially preventing their progression to heart failure, according to the researchers.
Pirfenidone is approved by the Food and Drug Administration to treat a lung condition called idiopathic pulmonary fibrosis, a scarring of the lungs that has no known cause. The drug also has been known for its heart-protective effects in a number of different animal models of heart attack. Researchers had assumed that pirfenidone’s protective action in the heart paralleled the reason it helps in lung disease. In the lungs, the drug slows the formation of scar tissue.
“That this drug also protects the heart is not new,” said first author Luigi Adamo, MD, PhD, a clinical fellow in cardiology. “But in our studies, pirfenidone didn’t physically reduce scar tissue in the heart. The scar tissue is still there, but somehow the heart works better than expected when exposed to this drug. It wasn’t clear why. So we set out to reverse engineer the drug to pick apart how it may be working. Since scar tissue was still present, we suspected inflammation was the main culprit in poor heart function after a heart attack.”
Adamo said that most immune studies of the heart have focused on other types of immune cells, including macrophages, T cell lymphocytes, neutrophils and monocytes. But he found no differences in the numbers of such immune cells in the injured hearts of mice that received pirfenidone versus those that didn’t. When he serendipitously measured B cells, however, Adamo was surprised to see a huge difference.
“Our results showing B cells driving heart inflammation was quite unexpected,” Adamo said. “We didn’t know that B cells have a role in the type of heart damage we were studying until our data pushed us in that direction. We also found that there isn’t just one type of B cell in the heart, but a whole family of different types that are closely related. And pirfenidone modulates these cells to have a protective effect on heart muscle after a heart attack.”
However, Adamo added that when the researchers removed these cells completely, not only was the heart not protected, the beneficial effect of the drug went away. So the B cells are not exclusively bad, according to the scientists. If they were, removing them completely would protect the heart.
“The protective effects of pirfenidone hinge on the presence of B cells,” Adamo said. “The drug may be working on other cells as well, perhaps directly or perhaps through the B cells. We’re continuing to investigate the details.”
Adamo said that pirfenidone has long been considered safe but that it can have side effects such as nausea and vomiting. He explained that researchers have not tried to improve upon the drug because no one understood precisely how it worked. Now that Adamo, Mann and their colleagues have identified B cell lymphocytes as the drug’s target, they can begin investigating ways to make it better. With the support of Washington University’s Office of Technology Management, the scientists have launched a startup company focused on designing a version of pirfenidone that maintains the drug’s ability to modulate B cell lymphocytes but that may be more tolerable for patients.






