Soluble electronics reliably monitor intracranial pressure
Using soluble circuits and wireless communication to monitor intracranial pressure may improve traumatic brain injury outcomes
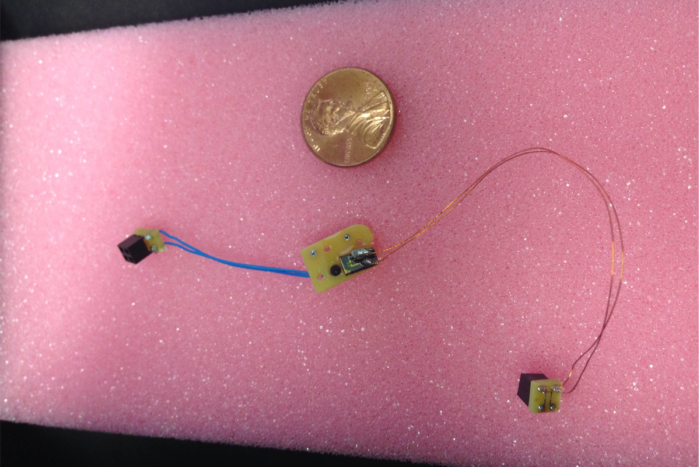
Absorbable implants such as these can monitor intracranial pressure.
As the “signature injury” of the wars in Afghanistan and Iraq, traumatic brain injury (TBI) has made the public aware that the moment of impact is just the start of the severe brain trauma story. Lifelong disability or death—primarily due to uncontrolled rise in intracranial pressure—may follow the initial injury, and the care given in the first 48 hours will often determine the outcome. In the United States alone, more than 53,000 individuals die annually because of TBI, contributing to 30.5% of all injury-related deaths.
That’s why monitoring intracranial pressure is an important component of TBI management at brain trauma centers throughout the US. But current techniques, which require surgical insertion of a conventional device and a second surgery later to remove it, temper the benefit by imposing surgical risks as well as additional cost.
“Innovations in transient electronics, the electronic materials that include biocompatible devices that dissolve in the body, can dramatically improve the way we assess intracranial pressure,” says Wilson Zachary Ray, MD, a Washington University neurosurgeon. “And, as a device reaches its useful lifetime, it will simply disappear—resorbed by the body. This is technically achievable today.”
Wireless dissolving devices in animal trials
Animal trials in the Ray laboratory have shown that fully biodegradable electronic devices with wireless capability give reliable pressure and temperature measurements. The project has now moved forward to animal safety trials.
Rory Murphy, MD, a neurosurgery resident in Ray’s lab, along with materials scientist Seung-Kyun Kang, PhD, and Dan Harburg, PhD, a bioengineer at the University of Illinois at Urbana/Champaign have partnered to drive the project, using silicon circuits, or chips, that dissolve in water or body fluids. Murphy said that the next goal is to make the wireless components of the system able to be absorbed as well.
Although silicon is fairly inert, it will react slowly in body fluids to form silicic acid. How long a chip survives in the body can be adjusted by changing the thickness of the silicon circuitry; the thinner the silicon, the shorter the lifespan of the device. The chips used in the animal trials are less than one-thousandth of the thickness of human hair and dissolve in one or two weeks.
Ray believes that intracranial sensors of this type that can be absorbed by the body can be incorporated into all neurosurgery disciplines, but most importantly in brain injury diagnosis, treatment and research. He even envisions a day when a medic on a battlefield or paramedic by a roadway will be trained to insert a minute device that will wirelessly transmit brain temperature and pressure while the patient is in transit to a brain trauma center. “Dr. Murphy has already developed a straightforward and quick insertion procedure in the animal models,” said Ray.
“Materials science has come a long way in creating sensors that are tolerated by the body as well as protected from it for specific durations,” says Ray. He adds that making these devices useful in medicine will require thinking in terms of the whole system – from processing to communication.






