Possible strategy identified for Charcot-Marie-Tooth disease, other disorders
Research leads to development of compounds to correct mitochondrial dysfunction
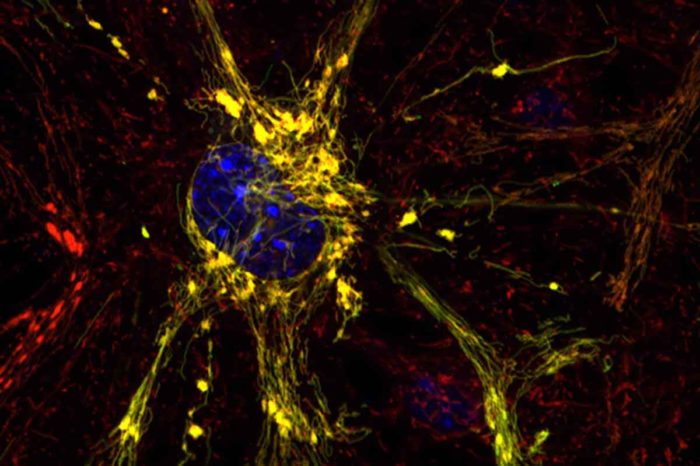 G. Dorn and A. Franco
G. Dorn and A. FrancoShown is a diseased neuron, with disease indicated by clumpy yellow mitochondria. Scientists at Washington University School of Medicine in St. Louis and Stanford University have designed small compounds that have the potential to correct mitochondrial dysfunction that leads to Charcot-Marie-Tooth disease and other conditions involving mitochondria, the cells' energy factories.
Charcot-Marie-Tooth disease is an inherited disorder that leads to a gradual loss of motor neurons and, eventually, paralysis. The condition is caused by genetic mutations that disrupts cells’ energy factories, called mitochondria. No drugs are available to slow or stop the progression of the disease, which affects nearly 3 million people worldwide.
However, in research slated for fast-track advance online publication Oct. 24 in Nature, scientists at Washington University School of Medicine in St. Louis and Stanford University report that they have designed small compounds that have the potential to correct the mitochondrial dysfunction that leads to Charcot-Marie-Tooth and other conditions involving mitochondria. The team designed the compounds after its work in mouse cells revealed a new understanding of the 3-D structure of a key protein that is disabled in the mitochondria of patients with the disease.
“This mitochondrial protein has never been targeted before,” said senior author Gerald W. Dorn II, MD, the Philip and Sima K. Needleman Professor of Medicine. “There are no drugs that work on this protein that is so important for mitochondrial function. We designed two compounds — one that activates and one that inhibits the function of this protein. We are working on testing them in mice with mitochondrial defects.”
Most people with Charcot-Marie-Tooth disease begin to see symptoms between ages 10 and 20. Patients with the condition have an average lifespan but slowly lose motor control, especially of the legs. Onset of symptoms before age 10 is associated with more severe disease, and such patients eventually may require crutches or a wheelchair.
The mitochondrial protein the researchers studied is called mitofusin 2. There’s a lot of interest in this protein because scientists think it also may have roles in many diseases, including diabetes and heart disease, that generally aren’t considered disorders of mitochondria. Mitofusin 2 governs whether two mitochondria are able to tether to each other and then fuse, exchanging genetic information, which is thought to be important for maintaining healthy mitochondria and, by extension, healthy tissues.
“In the past, scientists assumed mitofusin 2 was always active, always ready to tether to another mitofusin molecule and promote mitochondrial fusion,” Dorn said. “Our study now shows this is incorrect. Mitofusin 2 folds and unfolds, giving it active and inactive forms that either encourage or discourage tethering and the resulting fusion of mitochondria.”
Once Dorn and his colleagues, including co-author Daria Mochly-Rosen, PhD, of Stanford University, understood how mitofusin 2 changes shape, they were able to design small peptides that interact with the protein and drive it toward either an active or inactive state.
“We designed these molecules based on our new knowledge of mitofusin 2,” Dorn said. “My colleague, Dr. Mochly-Rosen, is a genius at designing this kind of small peptide drug. She looks at amino acid sequences and sees things I don’t see.”
One of the small molecules, dubbed GoFuse, forces mitofusin 2 into its active, healthy state, which encourages tethering and the resulting mitochondrial fusion. Conversely, the other small molecule, called TetherX, forces mitofusin 2 into its inactive state, which suppresses tethering and prevents fusion.
“The design of these peptide inhibitors was a challenge,” Mochly-Rosen said. “But it is always exciting when a basic research discovery leads to the design of a new drug that may eventually help patients who currently have no treatment options.”
Dorn said more work must be done to determine whether these small peptides will be effective in animal models of diseases. But the hope is that GoFuse, or a similar molecule, could encourage the mitochondrial tethering and fusion that is missing in Charcot-Marie-Tooth disease. If such tethering could be restored, it could prevent or delay the loss of motor neurons that gradually paralyzes many patients with this genetic disorder.
But the researchers see a potential use for the peptide inhibitors beyond Charcot-Marie-Tooth disease, such as reducing tissue damage that occurs when oxygen returns to the heart after a heart attack or to the brain after a stroke.
“Re-establishing oxygen flow is really important after a heart attack or stroke,” Dorn said. “But you also get a huge wave of cell death when oxygen suddenly returns to tissues of the body, such as the heart or the brain.”
The rush of oxygen back into tissues causes an influx of calcium into mitochondria that are tethered. Large amounts of calcium flowing into mitochondria causes water to rush in as well. Like an overfilled water balloon, the mitochondria burst, which kills the cell. But, Dorn speculated, if this type of tethering could be suppressed, it would prevent the sudden influx of calcium and protect mitochondria from being destroyed.
“These peptides are two sides of the same coin,” Dorn said. “Mutations that disrupt tethering cause a neurodegenerative disease. We would like to encourage tethering in that case. But there are other situations where tethering is destructive, and we would like the ability to interrupt it briefly and then go back to normal. We’ve shown these peptides can influence mitochondrial tethering in cells grown in the lab, and now we are working to test them in mouse models of disease.”






