Two-pronged immunotherapy eliminates metastatic breast cancer in mice
Suggests a new approach for treating breast cancers that have spread to bone
 Douglas V. Faget
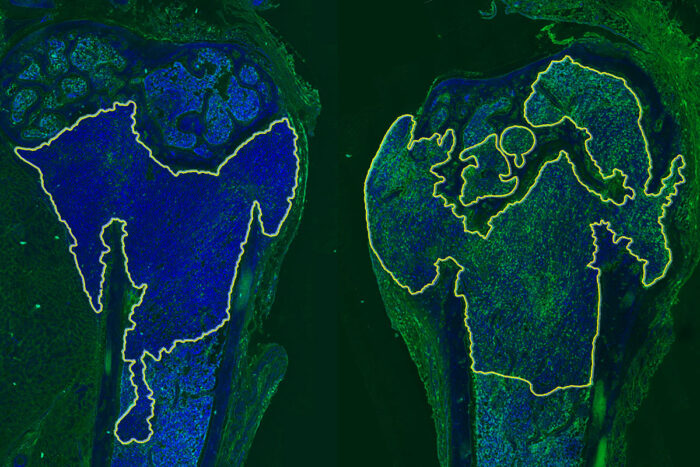
Douglas V. FagetResearchers at Washington University School of Medicine in St. Louis have identified a way to sensitize metastatic breast cancer that has spread to bone to immunotherapy. Shown are two bones from mice with metastatic breast cancer. Tumors are inside the yellow outline. The left side shows an untreated tumor. The right side shows a tumor treated with a p38MAPK inhibitor. The increased green color on the right indicates the tumor is more sensitive to immunotherapy.
Metastatic breast cancer has no cure and has proven stubbornly resistant to one of the most innovative and promising new cancer treatments: immunotherapy.
Now, researchers at Washington University School of Medicine in St. Louis have identified a way to treat the area surrounding breast tumors that have spread to bone so that such tumors become vulnerable to attack by the body’s immune system. When the researchers boosted the activity of certain immune cells, called T cells and macrophages, these immune cells worked together to clear metastatic breast tumors that had spread to the bones of mice, and continued to eliminate tumor cells that eventually returned.
The study is published March 8 in Cancer Discovery, a journal of the American Association for Cancer Research.
Macrophages are myeloid immune cells that attack cancer cells through the body’s innate immune response to general threats, such as tumors or viruses. Such macrophages further activate T cells by showing the T cells what they should be looking for, thereby harnessing the adaptive immune response as well. In this case, these macrophages present T cells with bits of recognizable tumor — called tumor antigens — from dead cancer cells, and the antigens direct the killing activities of T cells.
“After breast cancer has spread to other parts of the body, it becomes extraordinarily difficult to treat; current therapies can only try to slow it down,” said senior author Sheila A. Stewart, PhD, the Gerty Cori Professor of Cell Biology & Physiology. “About 70% of patients with metastatic breast cancer have tumors that have spread to their bones. Our study suggests we may be able to use two treatments — one to sensitize the myeloid tumor microenvironment to immunotherapy, and one to activate T cells — to target these bone metastases in a way that eliminates the tumor, prevents the cancer from returning and protects against bone loss in the process.”
Stewart, also a research member of Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, and her colleagues found that blocking a molecule called p38MAPK reprograms the tumor microenvironment to become more vulnerable to attack by the immune system, including by immune cells and signaling molecules called anti-tumor cytokines. While a p38MAPK inhibitor alone reduced tumor size, it didn’t eliminate the tumor entirely. So, the researchers investigated whether adding another therapy that activates T cells and boosts their ability to find and destroy the tumor cells could be more effective at eliminating the metastatic cancer cells.
Common immunotherapies — called immune checkpoint inhibitors — are often described as “taking the brakes off” immune T cells, ushering them into battle against cancer. In this case, Stewart described the new approach as “hitting the gas” on T cells, supercharging them to be more effective against the cancer.
The researchers investigated two models of human metastatic breast cancer in mice and found that the metastatic tumors were eliminated in mice that received a p38MAPK inhibitor and an immune therapy called an OX40 agonist, which binds and activates T cells. All these mice were still alive and tumor-free at least 80 days after treatment. Among mice receiving either of the two treatments alone, only about half of them were still alive 60 days after treatment.
“If we targeted the microenvironment to make it more sensitive to T cells and simultaneously hit the gas on the T cells, all of the mice were cleared of the metastatic tumors,” Stewart said. “If we came back after two weeks and challenged the mice again with the same tumor cells, their immune systems could clear those cells as well. It appears that their immune systems developed long-term memory and knew to attack those returning cancer cells. The mice look like they’re basically vaccinated against the cancer.”
Three different OX40 agonists are being investigated in phase 2 clinical trials for cancer, including breast cancer. And p38MAPK inhibitors have been investigated in a number of inflammatory disorders, including rheumatoid arthritis and chronic obstructive pulmonary disease.
“We are hopeful that our study will interest companies that make these drugs, so that we can work toward developing a clinical trial that could investigate this strategy in patients with metastatic breast cancer,” Stewart said.






