Understanding genetic influences on early outcomes after a stroke
Researchers are looking for genetic factors that may aid in the development of therapies that could improve stroke outcomes
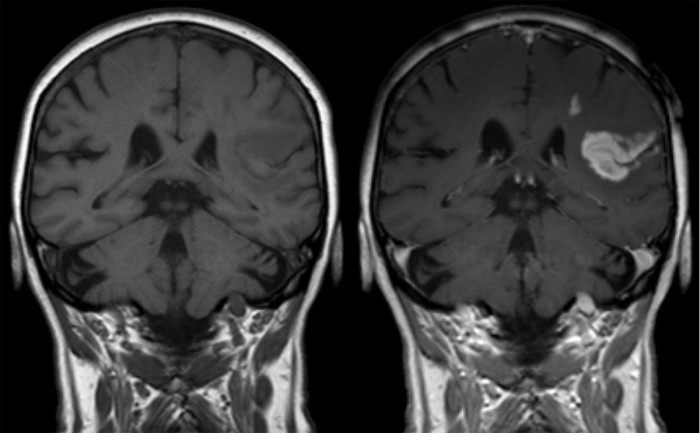
MRI of an ischemic stroke in the brain without (left) and with (right) contrast. Image courtesy of Hellerhoff, licensed under CC BY-SA 3.0 via Wikimedia Commons.
The first 24 hours after a stroke are critical; some patients show rapid improvement and others rapidly deteriorate. Neuroscientists are looking for genetic factors that may influence these events. If such factors exist, they could be valuable for guiding early treatment decisions and serve as targets for developing therapies to improve outcomes in the critical early hours.
“We want to pinpoint which genes influence the mechanisms that are really important for early neurological changes after stroke,” says Washington University neurologist and principal investigator Jin-Moo Lee, MD, PhD.
Examples of possible mechanisms that may lead to rapid improvement include restoring blood flow to a blocked artery and enhancing neuroprotective factors, which are released by the brain in response to injury. Mechanisms that might lead to rapid deterioration include bleeding and/or fluid accumulation in the brain and exaggerated inflammation.

To look for genetic factors, Lee and colleagues are collecting blood samples from ischemic stroke patients and analyzing their genomes. Study participants must, within six hours of stroke onset, present at the hospital and be assessed using the National Institutes of Health (NIH) Stroke Scale to quantify the degree of impairment caused by the stroke. Patients are re-evaluated with the same scale 24 hours after symptom onset.
“It’s so simple,” Lee says. “You have two time points to collect data to measure neurological change, and then you’re done. We want a streamlined and simple assessment in order to be able to do this in thousands of patients.”
In less than two years, the Washington University researchers, along with colleagues at Vall d’Hebron Hospital in Barcelona, Spain, have collected data from about 1,500 patients. They also are recruiting collaborating sites in Helsinki; Portland, Oregon; and perhaps Australia to participate in the study.
Lee’s colleagues at Washington University include psychiatry researcher Carlos Cruchaga, PhD, and emergency medicine physician Laura Heitsch, MD. Joan Montaner, MD, PhD, and Israel Fernández Cadenas, PhD, lead the team at Vall d’Hebron.
Seeking the brain’s protection
This genetic approach to finding neuroprotective targets in humans is new, Lee explains. Previously, researchers have used cellular or animal models, but their efforts have largely failed.
“The field has gone through more than 100 clinical trials to test efficacy of neuroprotective agents identified using animal models, which have all been negative,” Lee says. “But in our research, we are taking advantage of the natural variation in human genomes with the goal of finding targets relevant to human stroke. Rather than the bench-to-bedside approach, we are using a bedside-to-bench approach.”
The researchers have performed an interim analysis of the first 1,000 patients; although they did not identify any genetic changes with statistical significance across the data set, they found three that are nearly so. Lee’s hope is that by involving other clinical sites in the research—and therefore analyzing many more patients—they will be able to achieve statistical significance.
“If we find the genes that are involved in early stroke outcomes, the hope is that we will know where to target drugs to increase the likelihood of good outcomes and decrease the likelihood of bad outcomes,” Lee says. “We might also be able to develop diagnostic tests to predict outcomes, which could help guide therapies.”






