Understanding, treating pain, reducing opioid use, aim of $11.7 million grant
Funding from NIH HEAL Initiative
 Alex Chamessian/Maria Payne
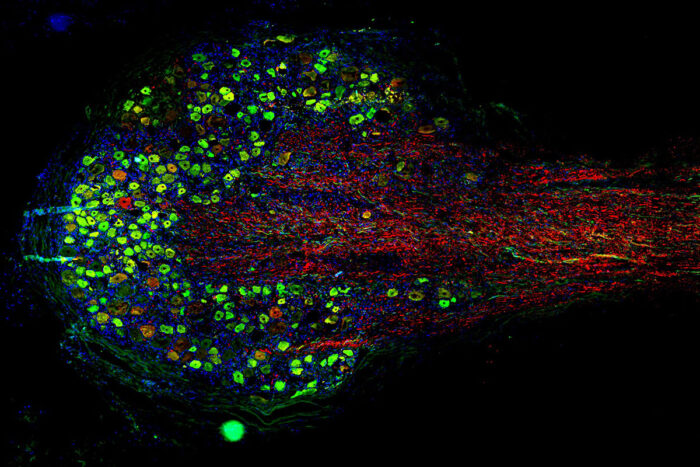
Alex Chamessian/Maria Payne Pictured is a microscopic view of a human dorsal root ganglion. The nerve tissue is stained to reveal the cell bodies (in green) and axons (in red) of neurons that sense and transmit pain sensations. Scientists at Washington University School of Medicine in St. Louis have received a five-year, $11.7 million grant to study human genes and nerve cells to better understand how cells transmit pain and to find new ways to treat it.
Researchers at Washington University School of Medicine in St. Louis have received a five-year, $11.7 million grant to study human genes and nerve cells to better understand how cells transmit pain and to identify new ways to treat it.
Washington University will be one of a handful of sites participating in the Precision Human Pain Network, a project funded by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH). The project is part of the NIH Helping to End Addiction Long-term (HEAL) Initiative. The goal of the massive effort is to identify science-based solutions to the opioid crisis. The opioid epidemic in the United States claimed the lives of more than 107,000 Americans in 2021. In addition, more than 50 million people in the U.S. suffer with chronic pain.
“Opioids are one of the very few medications that work well to manage acute pain, but they are ill-suited for treating chronic pain conditions,” said Robert W. Gereau, PhD, the Dr. Seymour and Rose T. Brown Professor of Anesthesiology and director of the Washington University Pain Center.
Gereau is the project leader for the Integrated Research Center for Human Pain Tissues (INTERCEPT) Pain Center at the School of Medicine. Scientists working on projects funded by the grant will collaborate with Mid-America Transplant, the organization that coordinates the region’s organ and tissue donation and transplantation. Mid-America Transplant will provide nerve tissue from donors for the studies.
“Our goal is to learn and detail which cell types and genes transmit pain signals and how they may change in the context of chronic pain,” Gereau explained. “We’ve done similar studies in the past in mice and rats, but we’ve learned that mice are not just small people. We need to advance the research by working in human tissue to truly understand and treat chronic pain so that we can improve people’s lives and reduce the need for prescription opioids.”
In previous studies involving rats and mice, Gereau and his colleagues focused on a cellular receptor that seemed key to the pain response, but they later discovered that human cells lacked that receptor. Tissue from human donors will help overcome those roadblocks.
The School of Medicine’s Integrated Research Center for Human Pain Tissues will be one of five to six such centers across the nation that eventually will be part of the Precision Human Pain Network. The Washington University center’s laboratories will be housed in the Neuroscience Research Building under construction on the Medical Campus.
The INTERCEPT Pain Center will be made up of three scientific projects and three supporting core groups. The first project, headed by Gereau and Bryan A. Copits, PhD, an assistant professor of anesthesiology, will focus on pain signals in the dorsal root ganglia, a group of cells responsible for transmitting sensory messages from pain-sensing receptors and neurons of the spinal cord, where the first stages of pain processing occur in the central nervous system. The researchers will compare the electrical properties of these cells to the patterns of gene expression in tissues from donors with and without a history of chronic pain.
A second project will use the most advanced technology to generate the first comprehensive single-cell atlas of human nerves, identifying how gene expression in the nerves is altered by pain on a cell-by-cell basis. In addition, the project will seek to identify the genetic underpinnings of peripheral neuropathy, one of the most common causes of chronic pain. Its principal investigators are Jeffrey Milbrandt, MD, the James S. McDonnell Professor, head of the Department of Genetics and executive director of the McDonnell Genome Institute; Aaron DiAntonio, MD, PhD, the Alan A. and Edith L. Wolff Professor of Developmental Biology; and Sheng Chih (Peter) Jin, PhD, an assistant professor of genetics and of pediatrics.
A third project will characterize gene expression in the human dorsal root ganglia with a technique called transcriptomics that involves the study of all of the RNA molecules within a cell. In addition, the project will focus on spatial proteomics, which deals with the organization of proteins within cells. The principal investigators on that project are Valeria Cavalli, PhD, the Robert E. and Louise F. Dunne Professor of Biomedical Research and a professor of neuroscience; and Guoyan Zhao, PhD, an assistant professor of neuroscience.
“Part of what we’ll do is catalog gene and protein expression from hundreds of donors over time to identify responses from tissue from otherwise healthy donors and compare that to tissue from those who had a history of chronic pain,” Gereau said. “By getting medical histories from the tissue donors, we can begin to map differences, not only involving those who had problems with chronic pain but also from those who used opioids, to see how those factors create differences in the pain response at the molecular, genetic and cellular levels.”






